Refine by
Contaminant Control Articles & Analysis: Older
10 articles found
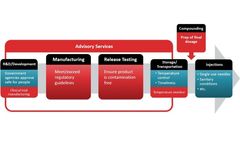
Part of Particle Measuring Systems’ role in making the world make the world cleaner, healthier & more productive As part of the Spectris PLC family of businesses, we aim to deliver value to society by providing solutions that make the world cleaner, healthier, and more productive. As part of Spectris, Particle Measuring Systems (PMS) supports this purpose in a variety of ways. One of ...
Here we will talk about when these actions should take place, who should perform them, and what documentation and other activities may be necessary to ensure that you enjoy the quality and contamination control benefits of a well-designed fill line with QbD. When QbD planning should occur as part of the fill line specification process. ...
This situation also leads to inefficiencies in the process and unnecessary movement can lead to material shedding and contamination control issues. Solutions for Set up and Process Interventions designed into fill lines designed with a PMS Quality by Design solution: Set-up – Solutions: The Set Up phase of production introduces the most risk of ...
The cleaning and disinfection of a pharmaceutical fill line & surrounding areas is a critical component of contamination control. We all think we are familiar with the principles of cleaning protocols, but it is deceptively easy to get this form of control wrong without applying the standards of QbD to the new equipment and facility at the ...
Below are several pitfalls to be aware of that might be thwarting your contamination control efforts if you are currently working with a piece of fill equipment that did not include QbD in the design phase. ...
These unintended outcomes include, but are not limited to, decreased productivity and product quality and increased cost to overcome contamination control hurdles. QbD from the Regulatory point of view emphasizes an understanding of the product and process fundamental enough to result in process control. ...
International experts met to share their knowledge and expertise of regulatory requirements and practical applications pertaining to contamination monitoring and control systems for aseptic processes and clean environments. ...
All starting materials should have minimal microbiological contamination. Your contamination control strategy should document the quality of the materials in terms of microbiological monitoring or other requirements. ...
All activities must be effectively controlled for contamination-free finished products. In order to build the PQS, a risk assessment is performed that encompasses the entire process. During the risk assessment, contamination is monitored to determine process needs, then broken down into individual elements and documented with the rationale for ...
This article offers some proactive steps for facilities managers and infection control personnel, to help minimize the risk of contamination during construction and renovation projects.Airborne DiseaseAt least 16 percent of hospital acquired infections are airborne in origin. ...