Particle Monitoring Articles & Analysis: Older
14 articles found
The determination of collagen molecular weight is a technique used to determine the molecular weight of collagen, a complex protein. Collagen is one of the richest proteins in animals, mainly found in the skin, bones, tendons and connective tissue. Due to its key role in cell structure and tissue regeneration, accurate determination of the molecular weight of collagen is of great significance to ...
Part of Particle Measuring Systems’ role in making the world make the world cleaner, healthier & more productive As part of the Spectris PLC family of businesses, we aim to deliver value to society by providing solutions that make the world cleaner, healthier, and more productive. As part of Spectris, Particle Measuring Systems (PMS) supports this purpose in a variety of ways. One of ...
Welcome to the world of liquid particle counting and syringe sampling! If you’re new to liquid particle counting, you might not know anything about how we know if liquids are clean. One method to determine a liquid’s cleanliness is syringe ...
Developing and manufacturing molecular diagnostics platforms and test kits for detecting human pathogens. Specializing in Antimicrobial Resistance Management and Hospital Associated Infections (HAIs), by reliable, cost-effective molecular diagnostic solutions for detecting Gastrointestinal Infections including most clinically relevant bacteria, parasites, viruses and antibiotic resistances. ...
The primary topics we explored are: Regulatory, Surround Areas, Cleaning, Process Interventions and Environmental Monitoring. Here we will talk about when these actions should take place, who should perform them, and what documentation and other activities may be necessary to ensure that you enjoy the quality and contamination control benefits of a well-designed fill line with ...
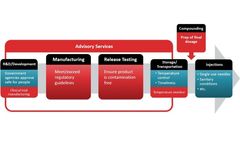
In the next blog in this series we will examine what Environmental Monitoring on a new fill line looks like with and without QbD implemented at the design phase. These examples come from real-world applications experienced by the Particle Measuring Systems’ Advisory Services Team. Our team has over 70 years of collective experience working in and advising ...
The cleaning and disinfection of a pharmaceutical fill line & surrounding areas is a critical component of contamination control. We all think we are familiar with the principles of cleaning protocols, but it is deceptively easy to get this form of control wrong without applying the standards of QbD to the new equipment and facility at the design phase. There are many things to consider from ...
The area surrounding an isolator/RABS pharmaceutical fill line can be a difficult area to control. There are many functions and moving parts that support the fill operations that occur in the controlled area just outside the controlled area that can greatly impact the process itself. Below are several pitfalls to be aware of that might be thwarting your contamination control efforts if you are ...
What does Quality by Design (QbD) in the design of clean manufacturing equipment and processes look like? This might be easier to visualize if we look at it from the perspective of the absence of it. Design and optimization based on only the engineering point of view, and not on understanding of the process, often leads to unintended impacts on the end-use of the equipment. These unintended ...
The following is the summary of a noteworthy discussion from the 2019 GMP Academy Master Course – Particle Measuring Systems, Rome, Italy The first GMP Academy Master Course took place from November 26 – 28 at Particle Measuring Systems (PMS) in Italy. International experts met to share their knowledge and expertise of regulatory requirements and ...
Before measuring particles in fluid, consider the method for delivering the sample fluid to the particle counter. For unpressurized fluid sources, the choices available depend on the sample fluid. Water provides the greatest range of options, while fluorinated chemistries such as HF can significantly restrict the options. Compatibility of the wetted surfaces with the sampling fluids will help you ...
Both Pharmaceutical Net and FacilityPro software provide data management from your particle counters, microbial monitors, and other sensors. The primary reasons you should upgrade to the FacilityPro Environmental Monitoring System: Data Integrity – Ensures accurate, unaltered data collection records Design Simplicity – Organizes ...
An alpha particle irradiator using 210Po radioactive sources was calibrated under specific conditions for cell irradiation. The energy spectrum of α-particles hitting the cell monolayer was measured by a surface barrier detector. The simulation of the results obtained with this detector was performed using the MCNPX Monte Carlo code with the same conditions used during the experimental ...
MFI™ for Parenteral Drugs USP Chapter 788 (Particulate Matter in Injections) describes physical tests to be performed for the purpose of enumerating subvisible particles within specific size ranges. Recently the FDA has published a Stimuli Article (reference USPC 30 (6) Pages 2272-2280) demonstrating that advances in apparatus and quality control merit reducing the permissible particle ...