- Home
- Companies
- Abionic SA
- Products
Abionic SA products
Acute Care Settings
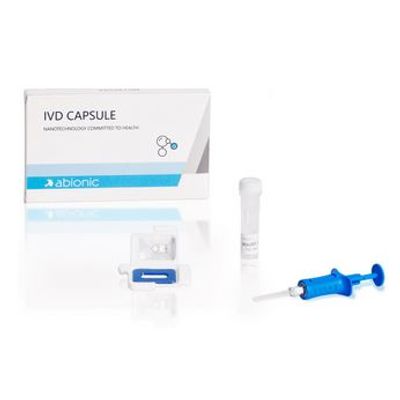
Abionic - Model IVD CAPSULE PSP - Rapid Single-Use in Vitro Diagnostic Test Kit
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the quantitative measurement of pancreatic stone protein (PSP) in blood. The test is intended to be used in conjunction with other clinical assessments and laboratory findings to aid in the early detection of nosocomial sepsis in adults.
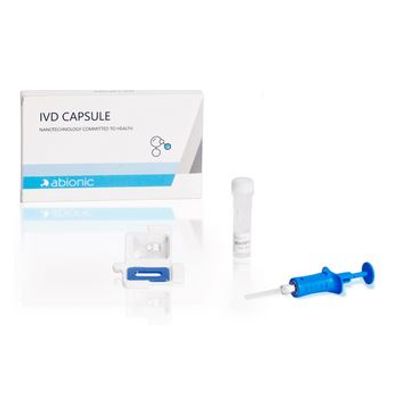
Abionic - Model IVD CAPSULE cSOFA - Rapid Single-Use in Vitro Diagnostic Test Kit
The IVD CAPSULE PSP is a rapid, single-use in vitro diagnostic test for the determination of Pancreatic Stone Protein (PSP) in blood for the calculation of the cSOFA score at the point of care. The cSOFA score is used to assess the severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in adults.
COVID19 Settings
Abionic - Model IVD CAPSULE COVID-19 ANTIGEN - Rapid Single-Use in Vitro Diagnostic Test Kit
The IVD CAPSULE COVID-19 is a rapid, single-use in vitro diagnostic test that measures the presence of a specific SARS-CoV-2 viral antigen in saliva. Abionic’s COVID-19 saliva test allows for ultra-fast screening of COVID-19 patients at the point-of-care with lab quality results.
Primary Care Settings
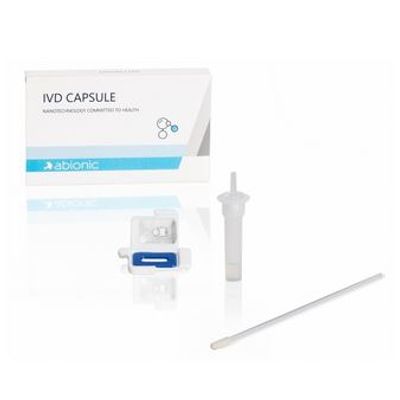
Abionic - Model IVD CAPSULE Ferritin - Rapid Single-Use in Vitro Diagnostic Test Kit
The IVD CAPSULE Ferritin is a rapid, single-use in vitro diagnostic test for the quantitative measurement of ferritin in capillary and venous whole blood, to aid blood diagnostic testing for iron deficiency in adults.
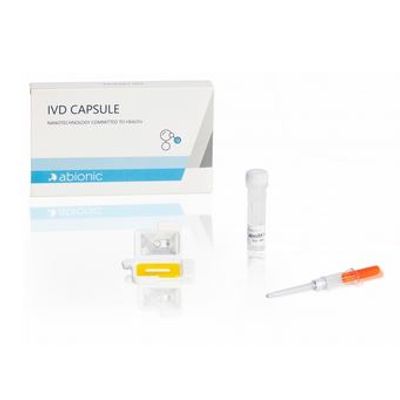
Abionic - Model IVD CAPSULE Aeroallergens - Rapid Single-Use in Vitro Diagnostic Test Kit
The IVD CAPSULE Aeroallergens is the first rapid, single-use in vitro diagnostic test for the quantitative determination of circulating immunoglobulin E (IgE) specific to the allergen components Phl p 1 + Phl p 5 (combined), Der p 1 + Der p 2 (combined), Alt a 1, Fel d 1 and Can f 1 in uncoagulated human capillary blood collected from the patient’s fingertip.
Other Products
abioSCOPE - Revolutionary in Vitro Diagnostic (IVD) System
Forcing molecules into a nanochannel, limiting the travel distance to a few hundred nanometres and reducing incubation time to 2 minutes.