- Home
- Companies
- ABK Biomedical Inc.
- Products
ABK Biomedical Inc. products
ABK - Embolic Microspheres
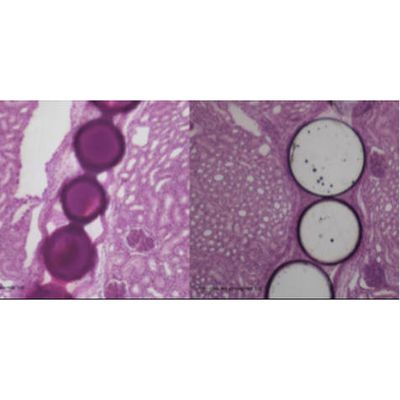
Radiopaque embolization device allowing direct visualization of the microspheres implant during the embolization procedure. FDA 510(k) clearance for the embolization of arteriovenous malformations and hypervascular tumors. A tailored radiopaque permanent embolization microsphere. Easi-Vue™ embolic microspheres are being developed in a range of microsphere sizes that are inherently radiopaque for direct visualization of the microspheres at the target site. Easi-Vue™ embolic microspheres were 510(k) cleared by the FDA in September 2022 for the embolization of arteriovenous malformations and hypervascular tumors.
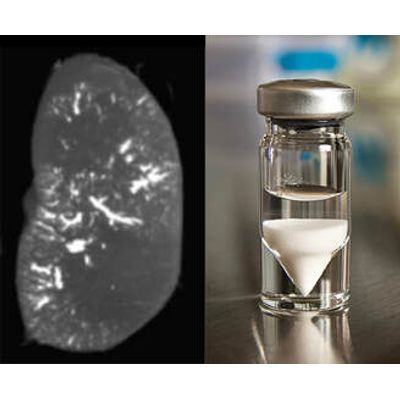
ABK - Microspheres
A novel technology for radioembolization made with a proprietary, radiopaque glass composition for in-procedure visualization. ABK Biomedical’s most advanced microspheres product in development is Eye90 Microspheres™. Eye90 microspheres™ is a novel technology for radioembolization made with a proprietary, radiopaque glass composition for in-procedure visualization via fluoroscopy, x-ray, CT and Cone Beam CT imaging modalities. Eye90 Microspheres™: An advanced new therapy designed to improve the targeting accuracy of Y90 microspheres, with the potential to provide in-procedure prognostic dosimetry for cutting-edge treatment of liver tumors.