- Home
- Companies
- Aptevo Therapeutics
- Products
Aptevo Therapeutics products
Technology
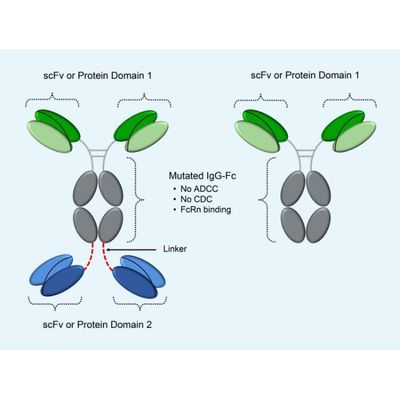
ADAPTIR - Modular and Flexible Technology
Demonstrated ability to produce monospecific and bispecific drug candidates; Leverages IgG1 Fc to form homodimeric bispecific molecules; IgG1-derived Fc can be mutated to ablate effector functions antibody-dependent cellcytotoxicity (ADCC) and complementdependent cytotoxicity (CDC), while maintaining original stability and half-life (confirmed by cell binding, cell function, SPRbased measurements of affinity, melting temperature) and good pharmaco-kinetic properties in preclinical studies; Binding domains are engineered from IgG variable regions, fragment libraries, receptors or ligands; Optimized to minimize proteolytic cleavage and post-translational modifications.
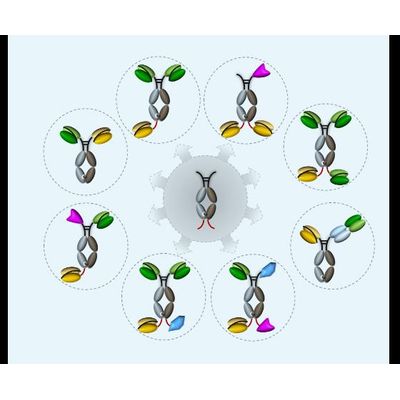
ADAPTIR-FLEX - Technology
ADAPTIR-FLEX technology extends advantages of ADAPTIR technology to create protein therapeutics with varied specificity and valency and potentially new modes of action.
Pipeline
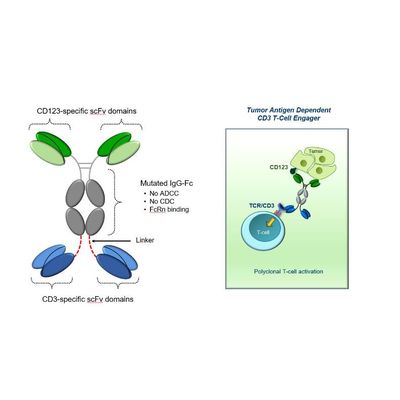
Aptevo - Model APVO436 (anti-CD123 x anti-CD3) - Optimized ADAPTIR Bispecific Antibody
APVO436 is an optimized ADAPTIR bispecific antibody candidate designed to redirect T-cell cytotoxicity through the dual targeting of CD3, a T-cell co-receptor that promotes cytotoxicity, and CD123, a cell surface receptor highly expressed in several hematological malignancies, including acute myeloid leukemia, acute lymphoblastic leukemia, hairy cell leukemia, myelodysplastic syndrome, and blastic plasmacytoid dendritic cell neoplasm. Aptevo is currently testing APVO436 in a Phase 1/1b open label clinical trial in patients with Acute Myeloid Leukemia (AML) and High-Grade Myelodysplastic Syndrome (MDS).
Aptevo - Model ALG.APV-527 - Bispecific Antibody
ALG.APV-527 is a bispecific antibody candidate targeting 4-1BB x 5T4 and is intended for tumor-directed treatment of solid cancers. It was built using Aptevo’s ADAPTIR™ bispecific technology platform and combines binding domains sourced from Alligator Biosciences’ ALLIGATOR-GOLD® human scFv library.
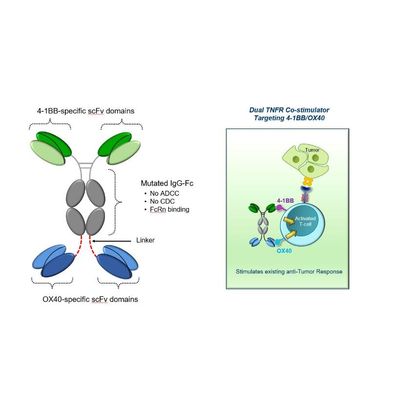
Aptevo - Model APVO603 - Dual Agonist Bispecific Antibody
APVO603 is a dual agonist bispecific antibody employing a novel mechanism of action to simultaneously target 4-1BB (CD137) and OX40 (CD134), both members of the TNF-receptor family. Dual targeting of 4-1BB and OX40 provides synergistic co-stimulation of T cells with the potential to amplify the cytotoxic function of activated T cells and NK cells, potentially leading to more robust anti-tumor responses.