- Home
- Companies
- AstraZeneca
- Products
AstraZeneca products
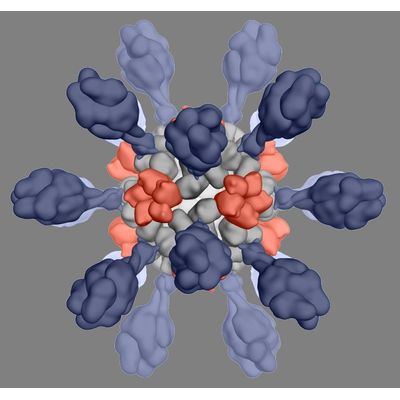
Icosavax - Model IVX-121 - RSV VLP Vaccine Candidate
IVX-121 targets respiratory syncytial virus (RSV), a major cause of viral pneumonia for which no vaccine has been FDA approved. IVX-121 incorporates a stabilized prefusion F antigen licensed from NIAID/NIH (DS-Cav1; Science 2019). RSV F is known to undergo major structural changes that allow viral entry into the host cell, and during that process, critical protective epitopes are lost. Protein design methods have stabilized prefusion F, leading to improved neutralizing responses in humans.
Icosavax - Model IVX-A12 - RSV/hMPV Bivalent VLP Vaccine Candidate
IVX-A12 is a bivalent combination of IVX-121 and IVX-241, a human metapneumovirus (hMPV) VLP vaccine candidate. IVX-A12 is being designed to target both RSV and hMPV in a single vaccine candidate. hMPV is being increasingly recognized as a major contributor to acute respiratory infection and pneumonia with rates of pneumonia (Clinics in Chest Medicine 2017) and hospitalization (JID 2012) similar to that of RSV and influenza. RSV, hMPV, and influenza seasons show high seasonal overlap and have similar clinical presentation; as such, hMPV is underdiagnosed and often mistaken for RSV or influenza given the similarity in clinical presentation. There are no FDA approved vaccines for hMPV. Similar to RSV, prospective cohort studies have shown that higher baseline hMPV nAbs are associated with reduced risk of hMPV symptomatic virus infection (Vaccine 2010), so the goal of vaccination is to increase hMPV nAbs.
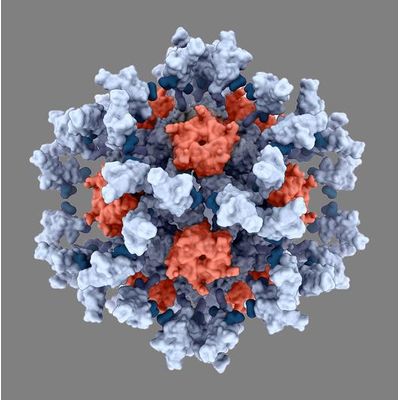
Icosavax - Model IVX-411 - SARS-CoV-2 VLP Vaccine Candidate
IVX-411 targets SARS-CoV-2, the coronavirus that causes COVID-19. Developed using cutting-edge structure-based design techniques at the Institute for Protein Design at the UW School of Medicine, IVX-411, our lead vaccine candidate for COVID-19, is a self-assembling protein nanoparticle that displays 60 copies of the SARS-CoV-2 spike (S) glycoprotein receptor-binding domain (RBD) in a highly immunogenic array.
Icosavax - Model IVX-421 - SARS-CoV-2 Beta (B.1.351) Variant VLP Vaccine Candidate
Icosavax has initiated development of IVX-421, a SARS-CoV-2 vaccine candidate that incorporates an RBD antigen with critical mutations found in the SARS-CoV-2 beta strain.
Oncology
Model AZD0466 Phase I - Inhibitor for Haematological Malignancies
Mechanism: BCL2/xL; Area under investigation: haematological malignancies; Date commenced phase: Q4 2019; Estimated Filing Acceptance: US: EU: Japan: China; Additional information: Partnered product. Molecule size: Small molecule; Status change.
Model AZD1390 Phase I - Glioblastoma
Mechanism: ATM inhibitor; Area under investigation: glioblastoma; Date commenced phase: Q2 2018; Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule Status change.
Model AZD4573 Phase I - Haematalogical malignancies
Mechanism: CDK9 inhibitor Area under investigation: haematalogical malignancies Date commenced phase: Q4 2017 Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule Status change: New to Phase II.
Cardiovascular, Renal & Metabolism
Model AZD2373 Phase I - Nephropathy
Mechanism: Podocyte health; Area under investigation: nephropathy; Date commenced phase: Q1 2020; Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule; Status change.
Model AZD2693 - Phase I - NASH
Mechanism: NASH resolution; Area under investigation: NASH; Date commenced phase: Q4 2019; Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule; Status change.
Model AZD3366 - Phase I - Cardiovascular Disease
Mechanism: CD39L3. Area under investigation: cardiovascular disease. Date commenced phase: Q4 2020. Estimated Filing Acceptance: Country:US, EU, Japan, China, Additional information: Molecule size: Small molecule. Status change: