- Home
- Companies
- CRScube Inc.
- Software
CRScube Inc. software
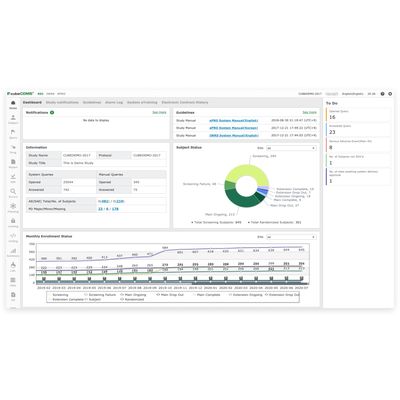
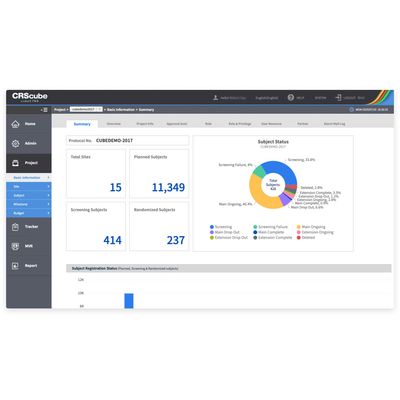
cubeCDMS - Recognized Compliant Solution
cubeCDMS® is a recognized compliant solution with the FDA`s 21 CFR Part 11. It ensures seamless accurate eCRF data collection with simple and efficient data management.
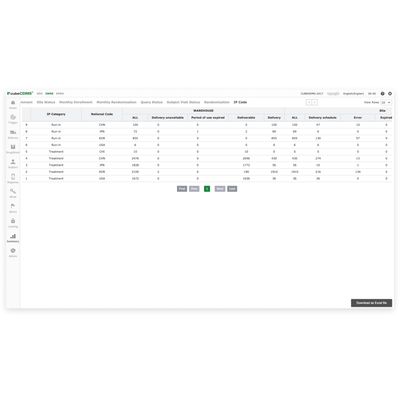
cubeIWRS - Solution for Drug Distribution and Delivery Logistics
cubeIWRS® is a powerful solution that manages all aspects of a study`s randomization, drug distribution, and delivery logistics. With cubeIWRS®, users have an intuitive, single platform that oversees randomization and drug supply management.
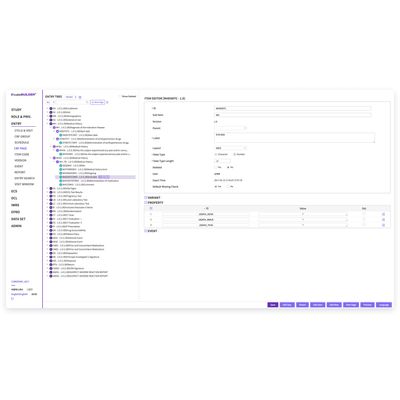
cubeBUILDER - eClinical Solution
cubeBUILDER® provides an intuitive interface for sponsors, CROs, and academic institutions to access and rapidly build cubeCDMS®, cubeIWRS®, and cubePRO® system environments to conduct clinical trials electronically and securely on the cloud. cubeBUILDER® is free, easy-to-learn, and easy-to-use.
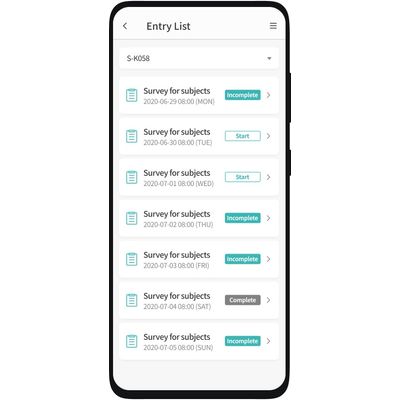
cubePRO - Mobile Application
cubePRO® is a mobile application used to collect ePRO (electronic Patient Reported Outcomes) data. Data is entered directly by participating patients which leads to a minimization in data reconciliation as the data is automatically synced with cubeCDMS®` database. cubePRO® is setup using cubeBUILDER® and can be equipped with edit checks which results in a reduction in errors and ensures that clean data is entered.
cubeCONSENT - eClinical Solutions
cubeCONSENT® provides a learning environment for subjects and ensures that the subject completely understands the trial prior to signing the Informed Consent Form (ICF). cubeCONSENT® is interoperable and integrated with the cubeCDMS® with subjects being automatically enrolled in cubeCDMS® upon signing the ICF.
cubeCTMS - Clinical Trial Management System for Real-Time Central Monitoring
cubeCTMS® is a clinical trial management system for real-time central monitoring allowing users to view and manage information on the progress of a clinical trial and milestone at a glance. Additionally, cubeCTMS® provides users with effective resource allocation and risk-based monitoring can be planned, taking into account risk areas identified as issue tracking. Conduct clinical trials with cubeCTMS®.
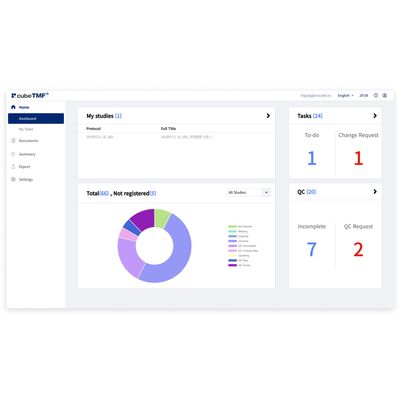
cubeTMF - Streamlines the Workflow and Management of Documents
In the paperless era, the clinical trial industry is changing. Until now, the Trial Master File (TMF) has consisted of traditional paper documents, images, or media organized in a binder stored in file cabinets. In accordance with FDA`s CFR 21 Part 11, we have developed cubeTMF to provide a validated system for a cohesive workflow for a truly paperless clinical trial. cubeTMF® streamlines the workflow and management of documents, images, and other digital content for clinical trials.
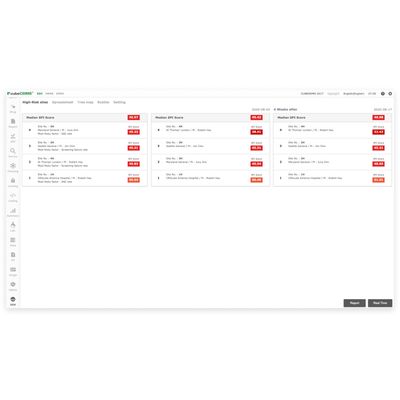
cubeRBM - Real-Time Monitoring Solution
cubeRBM® is a real-time monitoring solution that helps mitigate risk by planning and ensuring the quality of clinical trials by identifying, evaluating, monitoring and alleviating the risks that may affect the quality and safety of clinical trials. cubeRBM® collects the risks of each site participating in the clinical study in real-time through the EDC and evaluates and compares it with a unique index called SPI (Site Performance Index score). The data collected through cubeRBM® is visualized with charts and graphs, allowing users to identify clinically problematic sites through formal indicators.
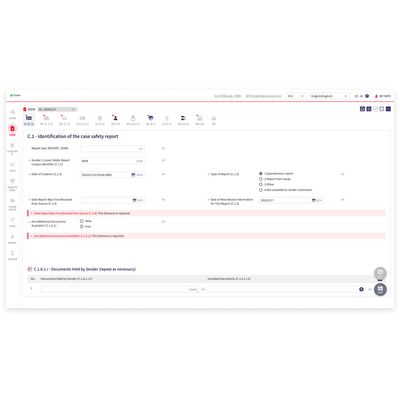
cubeSAFETY - Personnel, Processes and Management Systems
Pharmacovigilance requires a lot of resources such as personnel, processes, and management systems. cubeSAFETY® streamlines the Pharmacovigilance Reporting process; all the while being compliant with the regulations of regulatory agencies such as the FDA, EMA, CDE, MFDS, and PMDA.
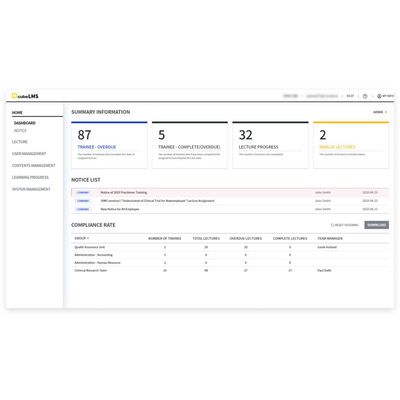
cubeLMS - Learning Management System
cubeLMS (Learning Management System) is a solution used for training and record management of internal personnel. cubeLMS features intuitive training scheduling, central real-time monitoring of training progress with metrics, viewing and exporting of training history for each staff/training course. cubeLMS also enables users to send announcements to specific users or teams to ensure timely training completion.