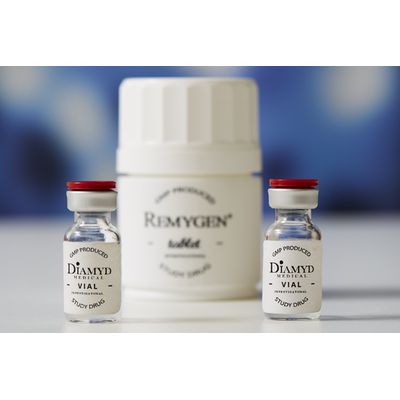
Diamyd Medical AB products
Remygen - Controlled Release Tablet
Remygen® is a controlled release tablet consisting of the active pharmaceutical ingredient GABA. The GABA substance is chemically synthesized in a well-controlled GMP process together with a global contract manufacturer. The manufacturing process is scalable and pediatric formulations with the same properties can be designed.
Diamyd - Antigen-Specific Intralymphatic Immunotherapy
Diamyd® is a suspension for injection containing the active pharmaceutical ingredient GAD65 mixed with the vaccine adjuvant Alhydrogel (alum). The human recombinant protein GAD65 is manufactured in insect cells in a well-controlled GMP process. The manufacturing process is developed for late phase clinical use. A new manufacturing facility is being set up by Diamyd Medical in Umeå, Sweden, with a first priority to receive the process technology for the manufacture of recombinant GAD65.