Galvanize Therapeutics, Inc. products
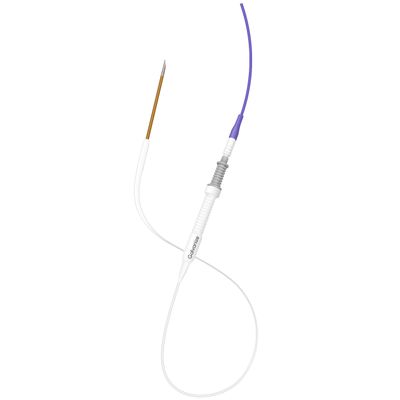
Galvanize Aliya - Aliya PEF System for Surgical Ablation of Soft Tissues
The Aliya PEF System is designed for the surgical ablation of soft tissues by delivering high voltage, short duration electrical energy. This targeted electrical energy alters the transmembrane potential, leading to the disruption of cellular homeostasis and inducing non-thermal programmed cell death. Utilizing technologies such as the Aliya Needle and the INUMI™ Flex Needle, the system enables precise ablation zones even in complex anatomical locations, minimizing collateral damage to surrounding healthy tissue. The non-thermal approach preserves the extracellular matrix, potentially activating an immune response that can complement existing treatment modalities. Though cleared by the FDA for soft tissue ablation, its application to immune response induction remains investigational and unproven for patient benefit beyond current indications.
Galvanize Aliya - PEF System for Soft Tissue Ablation
The Aliya PEF System is an advanced medical device used primarily for the surgical ablation of soft tissues, delivering high voltage, short-duration electrical energy to target cells. The system employs pulsed electric field technology to disrupt cellular homeostasis, leading to non-thermal programmed cell death while maintaining the integrity of the surrounding extracellular matrix. The system utilizes the INUMI Flex needle for endoscopic applications, providing a precise and predictable ablation zone with minimal interference from heat sinks. The technology shows promising synergies with standard cancer therapies by potentially activating an immune response through the release of antigens from destroyed tumor cells. However, its clinical efficacy beyond the ablation of soft tissue has not been fully established and is restricted by FDA clearance.
Galvanize RheOx - Investigational Device for Chronic Bronchitis Treatment
The RheOx System is an innovative medical device designed to alleviate symptoms associated with chronic bronchitis. It combines a specialized electrosurgical generator and a single-use catheter to deliver Pulsed Electric Field (PEF) energy non-invasively. This system targets and ablates abnormal mucus-producing cells within the airways, while preserving the integrity of the extracellular matrix, thus facilitating rapid epithelial regeneration. Initial clinical trials have demonstrated the system's feasibility and significant improvements in patient quality of life metrics, while maintaining a favorable safety profile. The system is particularly engineered for bronchial rheoplasty procedures, aiming to reduce mucus production and cough frequency significantly. Although the RheOx System is CE certified, it remains an investigational device in the United States and is subject to federal restrictions for investigational use only. Ongoing studies continue to explore its potential in managing chronic bronchitis symptoms.