- Home
- Companies
- Invicro, LLC
- Services
Invicro, LLC services
Solutions
Drug Discovery Services
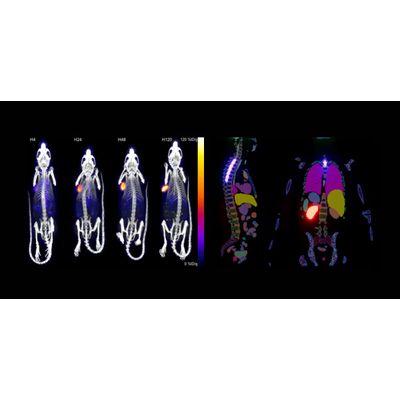
Invicro has a vast range of tools that help accelerate your drug discovery and development process. Our technology platforms support key decision-making in: Early discovery and screening. Candidate selection. First-in-human translation and early development. Late stage, multi-center determination of efficacy.
Late Phase Services
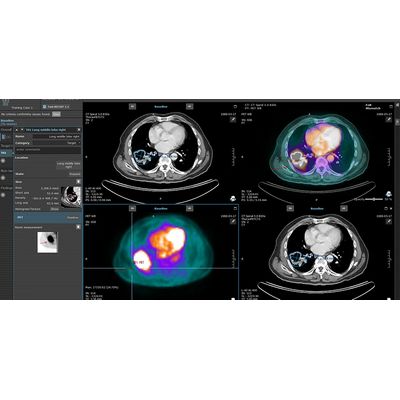
Invicro’s clinical capabilities span from early phase, first-in-human through late phase and post-approval studies. We focus on studies containing imaging and pathology-based endpoints requiring robust, independent assessments to ascertain drug efficacy. Our imaging core lab and CAP/CLIA lab-based pathology services solutions support various therapeutic areas including oncology, immuno-oncology, neurology (CNS), cardiovascular, musculoskeletal and gastrointestinal. Our experience comes from years of successful execution of Phase II-IV studies across the development spectrum led by a leadership team with an average of 25 years of industry experience.
Radiopharmaceutical Therapy & Theranostics Services
Radiopharmaceutical therapies represent an effective way to treat solid cancers by using tumor targeting small molecules, peptides or biologics to deliver a cytotoxic payload that induces DNA damage in tumor cells, while limiting damage to normal and healthy tissue. Streamlined development, evaluation and clinical translation of radiopharmaceuticals requires a partner that has extensive scientific, regulatory and operational expertise. Working with a CRO that provides a complete solution ensures data is delivered in a timely manner to make a go/no-go decision on your imaging or therapeutic candidates.
Advancing the PSMA-PET Standard of Care Services
Prostate cancer is the most common type of cancer (aside from skin) in American men with 13 out of every 100 being diagnosed with the disease during their lifetime.1,2Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is expressed in high levels in prostate cancer cells. Due to its location on the cell surface, PSMA is a popular target for imaging to identify the presence of prostate cancer and to deliver drugs directly to the cancerous cells3. A PSMA-PET scan is a powerful molecular imaging technique that can accurately localize prostate cancer and assess the level of a tumor’s PSMA expression. There are currently two USFDA and one EMA-approved PSMA-PET agents that are widely used in both clinic and trials. In early 2022, the USFDA approved the first targeted radioligand therapy, 177Lu-PSMA-61, for use in men with metastatic prostate cancer whose tumors overexpress the PSMA protein4.
Early Clinical Imaging Services
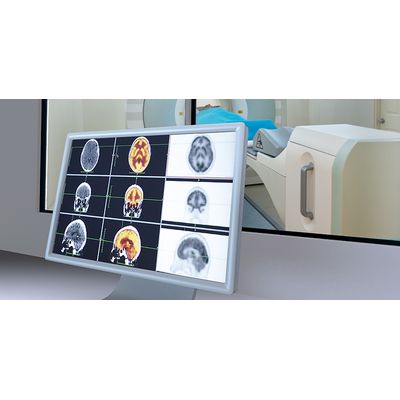
Invicro’s early clinical imaging services deliver decision-making data for drug development teams in Phases 0, I and II. We have many years of pharma industry experience, and this understanding of the drug development process helps us design and execute pathology and imaging studies so that the resultant data reduces risk in later phases of development. We own and operate clinics in London, UK and New Haven, CT allowing us to execute studies in both Europe and North America as required.