- Home
- Companies
- Maxim Biomedical
- Products
Maxim Biomedical products
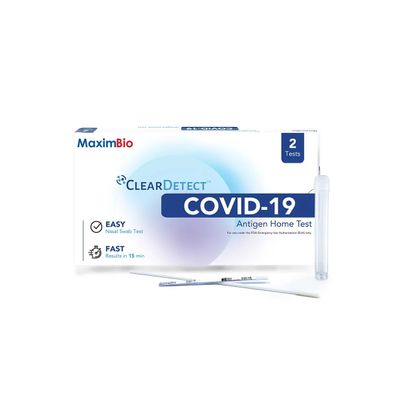
Clear Detect Covid 19 Antigen Home Test
The MaximBio ClearDetect™ COVID-19 Antigen Home Test is an antigen test that allows you to detect proteins from the virus that causes COVID-19. Made easy - An over-the-counter, no prescription needed nasal swab test you can do anywhere & anytime. Know your COVID-19 status in as little as 15 minutes! The MaximBio ClearDetect™ COVID-19 Antigen Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization. It is authorized for non-prescription self-use and/or as applicable for an adult lay user testing another person 2 years or older. The test is to be used in individuals within 5 days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
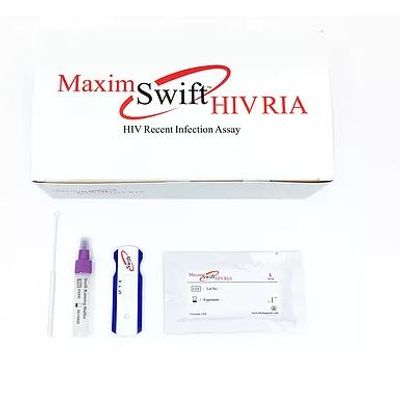
Maxim Swift - Model 92002 - HIV Recent Infection Assay (RIA) Kit
The Maxim Swift HIV Recent Infection Assay (RIA) is a single use qualitative immunoassay to detect the circulating antibodies to Human Immunodeficiency Virus Type 1 (HIV-1), Type 2 (HIV-2) and distinguish between recent and long-term infection in HIV-1/2. The assay is a point of care (POC) test intended for use with blood or serum/ plasma specimens. The Maxim Swift™ HIV RIA uses US CDC developed materials and is designed for surveillance purposes such as estimating HIV-1 incidence in a population, monitoring and evaluating HIV intervention programs, and recognizing those high-incidence populations so that prevention research, vaccine trials, and resources are most appropriately utilized. This product is for research use only and is not intended for use in diagnostic procedures. This product is for research purposes only.
Maxim Swift - Model 92002-C - HIV Recent Infection Assay (RIA) Controls Kit
The Maxim Swift HIV Recent Infection Assay (RIA) Kit Controls are quality control reagents for use only with the Maxim Swift™ HIV Recent Infection Assay (RIA) (P/N 92002). They are used to verify that the test kit reagents are working and that the test is performed correctly. The Maxim Swift™ HIV RIA uses US CDC developed materials and is designed for surveillance purposes such as estimating HIV-1 incidence in a population, monitoring and evaluating HIV intervention programs, and recognizing those high-incidence populations so that prevention research, vaccine trials, and resources are most appropriately utilized. Controls should be stored at 4-8°C for long-term storage (12 months) or at -20°C (24 months). This product is for research use only and is not intended for use in diagnostic procedures.
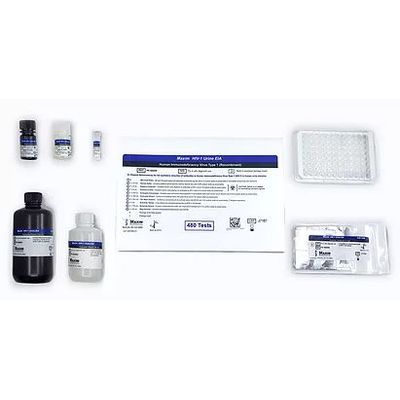
Maxim - Model 700000 - 480 Tests HIV-1 Urine Enzyme Immunoassay (EIA) Kit
Maxim`s FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 status can be made, specimens that are repeatedly reactive using this test should be further tested using Maxim`s additional, more specific FDA approved Cambridge Biotech Western Blot Kit.
Maxim - Model 92001 - Tests HIV-1 Limiting Antigen Avidity (LAg-Avidity) EIA Kit
The Maxim HIV-1 Limiting Antigen-Avidity EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit HIV-1 specific IgG populations with higher proportions of lower avidity IgG than those with long-term infections which typically exhibit higher proportions of higher avidity IgG. The Maxim HIV-1 LAg-Avidity EIA uses US CDC developed technology and is designed for surveillance purposes such as estimating HIV-1 incidence in a population, monitoring and evaluating HIV intervention programs, and recognizing those high-incidence populations so that prevention research, vaccine trials, and resources are most appropriately utilized. This product is for research use only and is not intended for use in diagnostic procedures.
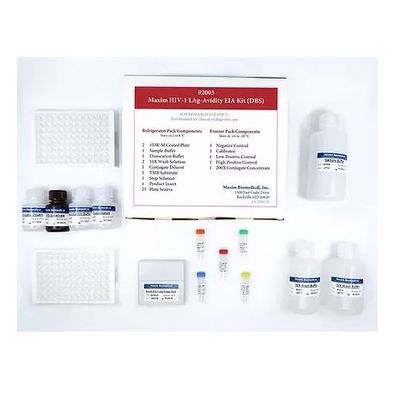
Maxim - Model 92003 - Tests HIV-1 Limiting antigen avidity (LAg-Avidity) DBS EIA Kit
The Maxim HIV-1 Limiting Antigen-Avidity Dried Blood Spot (DBS) EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit HIV-1 specific IgG populations with higher proportions of lower avidity IgG than those with long-term infections which typically exhibit higher proportions of higher avidity IgG. The Maxim HIV-1 LAg-Avidity DBS EIA uses US CDC developed technology and is designed for surveillance purposes such as estimating HIV-1 incidence in a population, monitoring and evaluating HIV intervention programs, and recognizing those high-incidence populations so that prevention research, vaccine trials, and resources are most appropriately utilized. This product is for research use only and is not intended for use in diagnostic procedures.