Medidata software
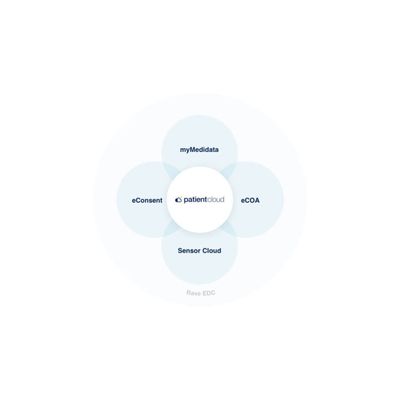
Patient Cloud
Medidata - Medidata Patient Cloud
Patient Cloud is a suite of powerful solutions that makes it simple and engaging for patients to participate in any clinical trial – so your trials are easier, faster, and produce better results. Built into the Medidata Clinical Cloud platform, Patient Cloud solutions combine Medidata’s leading clinical trial technology with unmatched patient centricity by design. Only Patient Cloud delivers the full range of tools to build scalable, flexible solutions at every level of onsite and decentralized trials, and our expert teams help you tackle problems creatively to find the most effective level of decentralization.
Medidata - Model eCOA - Clinical Outcome Assessment
Medidata eCOA (Clinical Outcome Assessment) is revolutionizing the way sponsors, CROs, and sites collect electronic data from patients, physicians, and caregivers. Available as an iOS or Android app or web-based solution, Medidata eCOA provides a flexible, intuitive model for capturing patient data that is designed to make it easier for patients to engage in clinical trials. Built as part of the unified Medidata Clinical Cloud™ platform, Medidata eCOA improves your study experience with flexible deployment options, a groundbreaking global instrument library, and dedicated services and support.
Medidata - Model eConsent - Innovative, Regulatory-Compliant, Patient-Friendly, Electronic Consent System for Clinical Trials
Medidata’s eConsent is an innovative, regulatory-compliant, patient-friendly, electronic consent system for clinical trials. Whether onsite or remote, Medidata eConsent automates the patient enrollment process and onboards patients directly into Rave EDC, improving overall consent tracking management, reducing informed consent errors, and easing the administrative burden for sites and study teams. It also enhances the patient experience with easy-to-understand clinical trial information while improving participant compliance and boosting patient engagement.
Rave Data Management
Rave EDC (Electronic Data Capture)
Medidata’s Rave EDC (Electronic Data Capture) is the most advanced, robust, and secure EDC system for clinical trial site, patient, and lab data capture and management. Rave EDC is the cornerstone of the Medidata Clinical Cloud® – the unified clinical research platform that connects processes, eliminates data reconciliation, and delivers cross-functional and cross-study data insights.
Rave Clinical Operations
Medidata - Rave Clinical Operations Software
Medidata AI
Medidata - AI Integrated Evidence
Medidata AI Integrated Evidence connects historical data from more than 23,000 cross-sponsor clinical trials and 7 million patient volunteers with real world data to deliver powerful insights and the necessary evidence clinical development leaders need to increase the probability of trial success. Artificial intelligence and data can help fuel new ways of working on clinical trials and beyond. Hear from Arnaub Chatterjee, Senior Vice President at Medidata AI as he discusses the importance of Evidence Generation in clinical trial planning on the MIT Business Lab podcast.
Medidata - AI Synthetic Control Arm (SCA)
Medidata AI Synthetic Control Arm (SCA®) offers the only external control arm created with cross-industry historical clinical trial data from 25,000+ clinical trials and 7 million patients. SCA can enable scientific research, cut costs and accelerate timelines in scenarios where a control group is hard to recruit or retain, such as rare or imminently life-threatening diseases with inadequate standard of care treatments, like some cancers.