- Home
- Companies
- OraSure Technologies
- Products
OraSure Technologies products
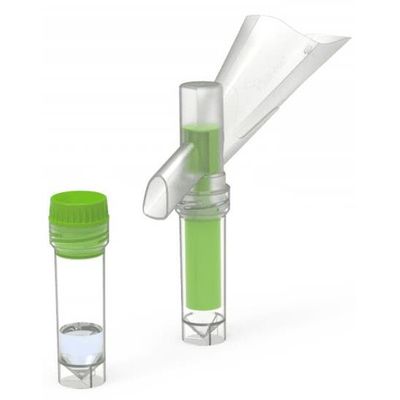
Colli-Pee - Model 20 mL - Volume Variants
Colli-Pee® 20 mL allows for standardized and volumetric urine collection of the first 20mL of urine flow. This has applications in infectious diseases and oncology. Collector tubes can be pre-filled with a preservative for different urinary analytes, improving transport and storage of urine at room temperature.
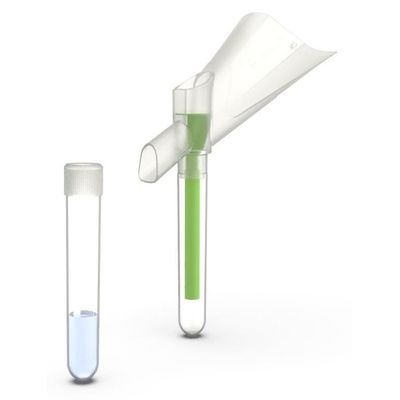
Colli-Pee - Small Volumes Tubes
Colli-Pee® Small Volumes tubes are compatible with high-throughput instruments and can streamline the pre-analytical process, shorten turnaround time, minimize errors as well as reduce costs. Collector tubes can be pre-filled with a preservative for different urinary analytes, improving transport and storage of urine at room temperature.
Colli-Pee - Colli-Pee Large Volumes for Detection and Monitoring of Cancers
For detection and monitoring of cancers, as well as new biomarker development, a larger volume of urine can be required. Additionally, Colli-Pee® Large Volumes supports multi-omics research. Collector tubes can be pre-filled with a preservative for different urinary analytes, improving transport and storage of urine at room temperature. Currently, the Colli-Pee Large Volumes variant is available for research use only.
Colli-Pee - Home-Based Collection Kits
Novosanis is working on Colli-Pee in a box for easy home-based urine collection. The kit allows postal delivery at home, next to postal shipment of the urine sample to the lab.
Colli-Pee - Urine Stability
Colli-Pee allows stabilization of collected urine: Urine contains useful and diagnostically relevant biomarkers to improve detection of infectious diseases, to uncover the molecular landscape of tumors in cancer as well as to research the human microbiome. Urinary analytes are susceptible to enzymatic and chemical degradation, supporting the needs for stabilization agents. Novosanis offers several preservatives that allow for sample stability next to shipping and storage at room temperature. A preservative would allow for at-home collection and facilitate large-scale recruitment.
Cryosurgery
OraSure Technologies, Inc. has announced the sale of its cryosurgical systems business to CryoConcepts, LP. The transaction includes the transfer of OraSure’s professional Histofreezer® product line and several private label cryosurgical products sold in the consumer market, along with related patents and trademarks, customer contracts and goodwill associated with the business. "We are pleased to have completed the sale of our cryosurgical systems business. The sale represents a key part of our innovation-driven growth strategy to prioritize our product portfolio and focus our resources on growing our core Molecular Solutions and Infectious Disease businesses through both organic growth and acquisition," said OraSurePresident and CEO, Stephen Tang, Ph.D.
Diagnostics & Testing - Infectious Disease Testing
ORAQUICK - Hepatitis C Virus Rapid Antibody Test Kit
The OraQuick HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole blood. Our simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
ORAQUICK ADVANCE - HIV-1/2 Rapid Antibody Test Kit
ORAQUICK RAPID HIV TESTING, ANYTIME, ANYWHERE, MADE EASY. THE ORAQUICK ADVANCE® RAPID HIV-1/2 ANTIBODY TEST DETECTS ANTIBODIES TO HIV-1 AND HIV-2 IN 20 MINUTES.
OraQuick - Ebola Rapid Antigen Test Kit
The OraQuick® Ebola Rapid Antigen Test has been granted De Novo 510(k) clearance by the U.S. Department of Health and Human Services for the following intended use: The OraQuick® Ebola Rapid Antigen Test is an in vitro diagnostic single-use immunoassay for the qualitative detection of antigens from viruses within the Ebolavirus genus but does not differentiate between these viruses. Testing with the OraQuick Ebola Rapid Antigen Test must only be performed when public health authorities have determined the need for this test. Testing for Ebola Virus Disease (EVD) must be performed in accordance with current guidelines provided by the appropriate public health authorities that address appropriate biosafety conditions, interpretation of test results, and coordination of testing, results and patient management with public health authorities.
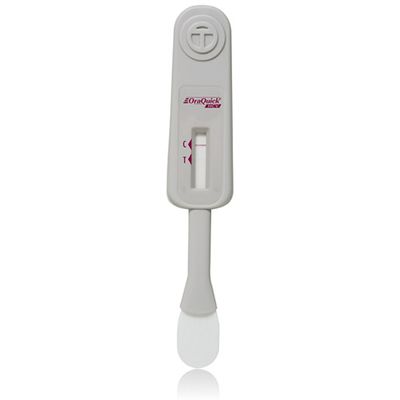
Oraquick - Home Hiv Test
The OraQuick® In-Home HIV Test is the first and only oral fluid rapid over-the-counter (OTC) HIV test approved in the U.S. It can detect antibodies to both HIV-1 and HIV-2 with an oral swab, providing a confidential in-home testing option with results in as little as 20 minutes. The test kit comes with extensive resources and support, including access to a "live" toll-free customer support center and comprehensive consumer website.