- Home
- Companies
- Parametrics Medical
- Products
Parametrics Medical products
Orthobiologics
Parametrics - Model Demineralized Bone Matrix (DBM) - Bone Grafts
Demineralized bone matrix (DBM) is decalcified allograft bone with osteoinductive potential. The demineralization process exposes growth factors, such as bone morphogenetic proteins (BMP), trapped by minerals. Growth factors recruit immature cells and stimulate those cells to develop into active bone-forming cells called osteoblasts.
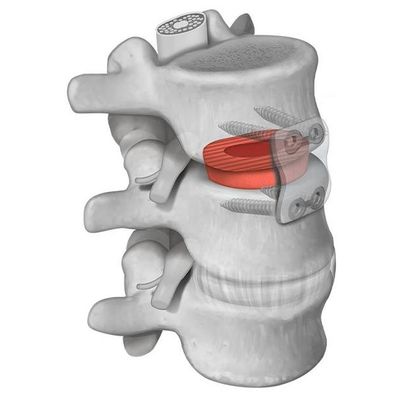
Parametrics - Model Machined Spacers - Bone Grafts
Machined interbody spacers provide a natural osteoconductive option for spine fusion. The allograft bone is precision machined to provide an optimal fit and stability between the allograft and adjacent vertebral bodies. Parametrics Medical offers spacers in various profiles, footprints, and heights.
OsteoSource - Void Filling Bone
OsteoSource™ allograft bone particulates have osteoconductive properties and are well suited to fill bone structure defects. Osteoconductive allografts act as a scaffold for the growth of natural bone. Over time, the natural bone replaces the donor’s bone. OsteoSource™ eliminates the need for autograft bone, associated with donor site morbidity, longer surgery time, and increased recovery time.
EnduriFuse - Advanced Bone Grafts
EnduriFuse is allograft bone containing viable bone-derived cells. This innovative graft contains properties ideal for the growth of natural bone: An osteoconductive three-dimensional scaffold with cortical and cancellous components. A demineralized bone scaffold with osteoinductive potential containing growth factors that recruit immature cells and stimulate those cells to develop into active bone-forming cells called osteoblasts. Bone-derived cells to support the osteogenic process. Osteogenesis is the growth of natural bone from preexisting living cells.
Parametrics - Synthetic Bone Void Fillers
Synthetic bone void fillers are safe, biocompatible, and easy to use. These resorbable biomaterials are based on nano-crystalline hydroxyapatite (HA) and purified collagen technologies. These products are engineered to optimize the resorption rate. Our granules are composed of 60% HA and 40% β-Tricalcium Phosphate (TCP). The formulation provides optimal osteoconduction, and the composition mimics that of human cancellous bone. Our strips are a combination of granules embedded in a fibrillar bovine collagen matrix. Once rehydrated, the foam is flexible. Our bioactive glass foam strips are sterile bone grafts composed of highly purified fibrillar Type I bovine collagen, bioactive glass granules, and HA/TCP granules. It functions as an osteogenic stimulus to which the patient’s bone marrow can be added before implantation.
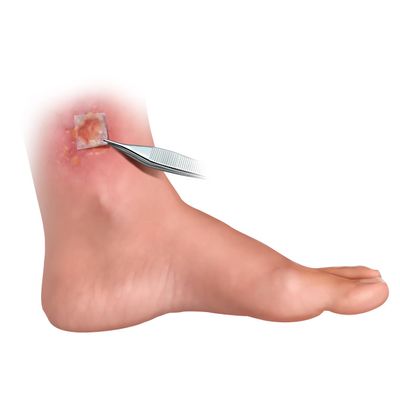
Coll-e-Derm - Model RT - Prehydrated Human Acellular Dermal Matrix
Coll-e-Derm RT is a prehydrated human acellular dermal matrix that supports integration, cellular repopulation, and revascularization. Coll-e-Derm may be used in numerous clinical applications, including but not limited to the in-office treatment of diabetic foot ulcers and venous leg ulcers.
Restorigin - Model Sx - Amniotic Tissue
Restorigin Sx amniotic membranes are procured through voluntary donation from scheduled cesarean procedures of full-term, live births. Amniotic membranes are applied as a tissue barrier to help provide mechanical protection while supporting healing with endogenous growth factors. Restorigin Sx may be used as a barrier in numerous surgical applications, including but not limited to spine and neurosurgery. Restorigin Sx amniotic membranes are stored at room temperature with a 5-year shelf-life, require no up-front preparation, and hydrate rapidly in the surgical site.
Wound Care
Restorigin - Amniotic Membranes
Restorigin amniotic membranes are procured through voluntary donation from scheduled cesarean procedures of full-term, live births. The natural properties of amniotic tissue provide mechanical protection and growth factors to aid in the management of diabetic foot ulcers and venous leg ulcers. Restorigin is a dual layer amnion that offers the flexibility of placing either side toward the wound, adheres naturally to the patient’s tissue without sutures or other fixation, and is stored at room temperature with a 5-year shelf-life.
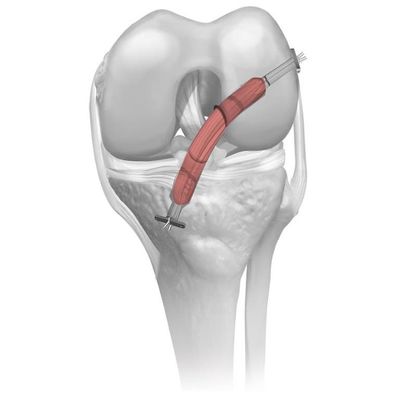
Sports Medicine - Allograft Tendons
Coll-e-Strong - Allograft Tendons
Coll-e-Strong allografts are Parametrics Medical’s trademarked electron beam sterilized tendons. This innovative processing methodology achieves a sterility assurance level (SAL) of 10-6 without the use of gamma irradiation. Coll-e-Strong grafts provide Parametrics Medical’s best option of sterility, safety, and strength for surgeons and patients.
Sports Medicine - Cartilage
Parametrics - Meniscus Cartilage
The biomechanical role of the meniscus is load distribution and stability in the knee joint. Parametrics Medical’s allograft meniscus with hemi plateau bone block is used in meniscal allograft transplant procedures and one of the few viable treatment options for the young meniscectomized knee. Our allograft meniscus are aseptically processed. No irradiation is used in processing or after packaging.