Profusa, Inc.
- Home
- Companies
- Profusa, Inc.
- Software
1 software found
Profusa, Inc. software
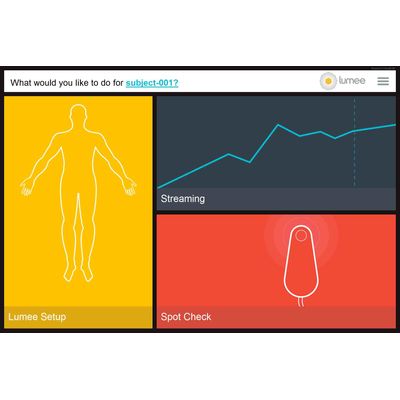
Profusa Lumee - Monitoring Tissue Oxygen Platform in Peripheral Arterial Disease Platform
Lumee™ Oxygen Platform is CE Marked for use in the European Union and all countries that accept the CE Mark. Caution: Investigational Device Limited by Federal (or United States) Law to Investigational Use. Profusa’s first clinical offering, the Lumee™ Oxygen Platform, is designed to report reliable tissue oxygen levels at various regions of interest, both acutely and long-term. Potential applications include monitoring of compromised tissue, such as peripheral artery disease that results in narrowing of blood vessels and reduced blood flow to the lower limbs; chronic wounds (diabetic ulcers, pressure sores) that do not heal properly; and reconstructive surgery.