- Home
- Companies
- Pulmonx Corporation
- Products
Pulmonx Corporation products
Zephyr - Minimally Invasive Bronchoscopic Procedure
The Zephyr Endobronchial Valve was granted a “breakthrough medical status”20 by the FDA and is indicated for bronchoscopic treatment of patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation (CV). The Zephyr Valve is an implantable device used to occlude all airways feeding the hyperinflated lobe of a lung that is most diseased with emphysema.
Technology
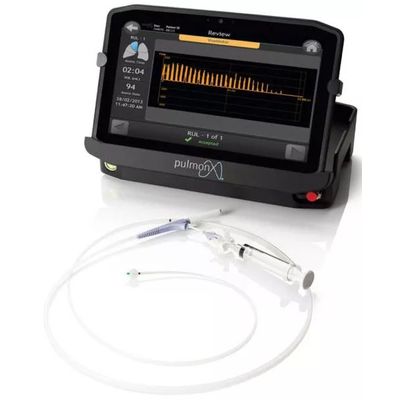
Chartis - Pulmonary Assessment System
The Chartis® Pulmonary Assessment System is a proprietary balloon catheter and console with flow and pressure sensors used to assess the presence of collateral ventilation (CV). It is a physiological measure of collateral ventilation.
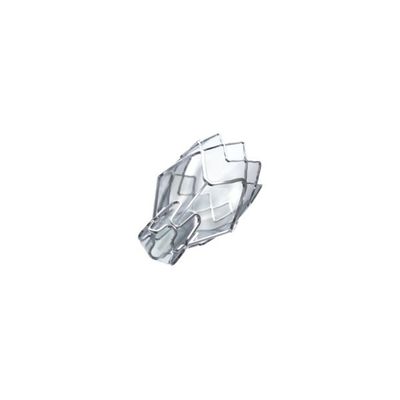
Zephyr - Endobronchial Valve
The Zephyr Endobronchial Valve is an implantable device used to occlude all airways feeding the hyperinflated lobe of a lung that is most diseased with emphysema.
Zephyr - Endobronchial Delivery Catheter (EDC)
Sizing and Placement in One Step: Easy to use. Quick and accurate sizing of the length and width of the airways. Equivalent catheter sizes to valve sizes. Available in a “J” configuration to allow the bronchoscope access to more angulated airways.