- Medical and Surgical Equipment - Ambulation and Equipment Management
- Medical and Surgical Equipment - Bed Frames
- Medical and Surgical Equipment - Cleaners and Disinfectants
- Medical and Surgical Equipment - Emergency Patient Transport
- Medical and Surgical Equipment - Stryker ENT product
- Medical and Surgical Equipment - Image Guided Therapies
- Cranial Neurotechnology
- Neurotechnology - Cranial - Cranial Access and Decompression
- Neurotechnology - Cranial Navigation
Stryker products
OrthoSensor - Model Verasense - Sensor-Assisted Total Knee Arthoplasty
Quantify Soft Tissue Balance and Customize Implant Position; Imbalance of soft tissues and improper position of the knee implant can result in pain, stiffness, limited range of motion and instability in the knee. VERASENSE enables surgeons to quantify ligament balance and customize implant position by giving them real-time, evidence-based data during primary and revision total knee arthroplasty (TKA). This disposable, intelligent device delivers data wirelessly to an intra-operative monitor that enables surgeons to make informed decisions regarding implant position and soft-tissue releases to improve knee balance and stability through the range of motion. This monitor – the LinkStation MINI – displays intraoperative data on a high definition tablet screen that occupies minimal space in the OR.
OrthoSensor - Model Verasense - Compatible Knee Implant Systems
OrthoSensor, Inc. is proud to have partnered with leading joint replacement companies Smith & Nephew, Stryker and Zimmer Biomet, allowing VERASENSE to be utilized in more replacement procedures around the world. VERASENSE is compatible for use with the following knee systems: Smith & Nephew Legion® and Journey II®, Stryker® Triathlon®, and Zimmer Biomet Persona®, NexGen® and Vanguard®.
Medical and Surgical Equipment - Ambulation and Equipment Management
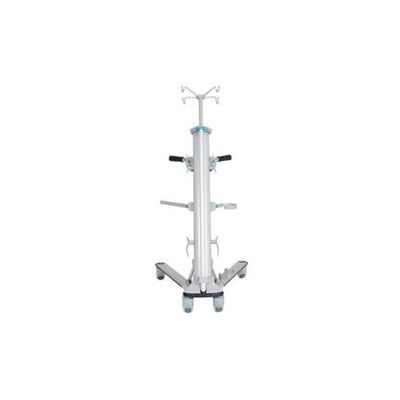
Stryker - Model IVEA - IVEA Equipment Management Tool
It’s estimated that 76% of patient ambulation orders are not met1, and that patients spend more than 96% of their hospital stay lying in bed or sitting2. Yet early and frequent mobility is crucial for patients on their road to recovery. IVEA’s features provide a simple, safe, and efficient solution to this problem, helping caregivers shorten the time it takes to ambulate a patient by providing them with a streamlined equipment management tool designed to replace the traditional IV pole.
Medical and Surgical Equipment - Bed Frames
Stryker - Stryker Critical Care Beds
Helping patients who need immediate interventions.
Medical and Surgical Equipment - Cleaners and Disinfectants
Auto - Model 62 - Medical and Surgical Cleaning Chemistries
Your tools need to do more than perform - they need to last. As a worldwide leader in power tool innovation and medical instrumentation, we`re well equipped to provide you with cleaning chemistries that help maximize your instruments` lifespan while minimizing your associated instrument costs.
Medical and Surgical Equipment - Emergency Patient Transport
Stryker - Model 6390 - Powered Cot Fastener Load System
Our Power-LOAD cot fastener improves operator and patient safety by supporting the cot throughout the loading and unloading process.
Medical and Surgical Equipment - Stryker ENT product
NasoPore - NasoPore Bioresorbable Basal Dressing
Nasal dressings providing the hemostasis, support and stability your patients and procedures require.
Medical and Surgical Equipment - Image Guided Therapies
Stryker - Dekompressor Disc Removal System
Disc diagnosis and therapy for back pain caused by symptomatic discs.
MultiGen - MultiGen Radiofrequency Generator for Cannulae or Electrodes
The MultiGen 2 Radiofrequency Generator heats a variety of cannulae and electrodes. Intelligently recognizing the electrode chosen, the MultiGen 2 RF Generator heats accordingly and automatically differentiates whether or not the procedure is monopolar.
Cranial Neurotechnology
Stryker - Model DirectInject - Cranial Closure On-Demand HA Cement
The first and only on-demand HA cement, redefining ease-of-use in cranial closure. A self-setting, calcium phosphate cement intended to repair neurosurgical burr holes, contiguous craniotomy cuts and other cranial defects not intrinsic to the stability of the bony structure. It is also intended for augmentation or restoration of bony contour in the craniofacial skeleton to include the cranial and zygomatic bones. DirectInject is intended to repair cranial defects with a surface area of 4 cm2 or less and is indicated for patients in whom skeletal growth is complete. You can use it in patients with surgically created bone defects.