Sublimed products
actiTENS
actiTENS - Medical Device for Relieving Chronic Pain
actiTENS is the product of a collaboration between engineers from the CEA in Grenoble and the Head of Service of the Pain Evaluation and Treatment Centre at the University Hospital Centre in Grenoble. actiTENS is a transcutaneous electrical nerve stimulation (TENS) medical device designed to manage chronic pain in adults. It is a drug-free medical technique that aims to relieve pain via electrical pulses targeting muscular fibre or nerves via electrodes placed on he skin. This pain treatment method is scientifically recognised and validated. The cases currently used are rigid, bulky, equipped with long cables, worn on a belt or around the neck, producing real physical and psychological discomfort for patients. actiTENS has been designed to improve the user experience and overcome the inconveniences of present-day TENS. CE 2797 actiTENS bénéfice du marquage CE.
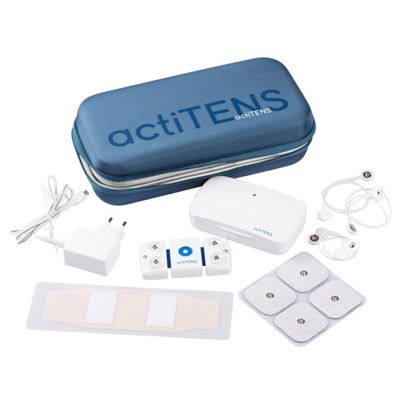
actiTENS - Model SMB1AA007 - Standard Kit
The actiTENS Standard Kit is compound of : an actiTENS, a charging box with power supply, a pack of 4 electrodes of 50x50mm, a pack of 2 adhesive support and 2 packs of 2 40cm Lead-wires, a quickstart, an instruction notice. Reference: SMB1AA007.
actiTENS - Model SMB1AB001 - Adhesive Media
The adhesive media actiTENS are reusable several times. The number of uses varies according to skin type, climate and precautions taken during use and storage. They have been tested for 7 to 10 collages / takeoffs. For several sessions of stimulation during the day, it is advisable to keep the adhesive support stuck on the skin between two sessions to improve its longevity. The lifetime of the adhesive backing is estimated at one week of treatment under these conditions.
actiTENS Electrodes
actiTENS - Model SMB1AC003 - Electrodes 50x50mm
actiTENS are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. They have been tested for 30 to 50 collages/takeoffs. It’s nevertheless advisable to change your electrodes every two weeks.
actiTENS - Model SMB1AC004 - Electrodes 50x90mm
actiTENS electrodes are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. They have been tested for 30 to 50 collages / takeoffs. It is nevertheless advisable to change your electrodes every two weeks.
actiTENS - Model SMB1AC002 - Electrodes 50mm
actiTENS electrodes are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. They have been tested for 30 to 50 collages / takeoffs. It is nevertheless advisable to change your electrodes every two weeks.
actiTENS - Model SMB1AC001 - Electrodes 32mm
actiTENS electrodes are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. They have been tested for 30 to 50 collages / takeoffs. It is nevertheless advisable to change your electrodes every two weeks.
actiTENS - Model SMB1AD002 - Lumbar Electrode
The lumbar electrode actiTENS is reusable several times. The number of uses varies according to skin type, climate, and precautions taken during use and storage. It has been tested for 30 collages / takeoffs. For several stimulation sessions during the day, it is advisable to keep the lumbar electrode stuck on the skin between sessions to improve its longevity.
actiTENS - Model BST11 - Electrodes Hypoallergenic Sensitive Skin 45x45mm
The hypoallergenic actiTENS electrodes are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. It is nevertheless advisable to change your electrodes every two weeks. This device is intended to be used on a single patient.
actiTENS - Model BST13 - Electrodes Hypoallergenic Sensitive Skin 45x95mm
The hypoallergenic actiTENS electrodes are reusable. The number of uses varies according to skin type, climate, and precautions taken during use and storage. It is nevertheless advisable to change your electrodes every two weeks. This device is intended to be used on a single patient.