Medical Device For Cognitive Assessment Testing Articles & Analysis: Older
13 news found
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the expansion of its service offerings to include the Bacterial Reverse Mutation Test (Ames) service. This service provides manufacturers and researchers a rapid, cost-effective, and robust method for assessing the mutagenic potential of new chemicals and leachable substances from ...
BySTEMart
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announces the expansion of its medical device testing capabilities and introduces Balloon Catheter Testing Services for the development of safe and effective medical devices. These reliable testing solutions will meet the evolving needs of the medical device industry and help manufacturers ...
BySTEMart
Protheragen-ING Lab, a well-known and reputable Good Laboratory Practices (GLP) service provider, has recently unveiled its cutting-edge medical device services. With a track record of excellence and reliability in providing high-quality services to the pharmaceutical and biotechnology industries, Protheragen-ING Lab is now expanding its offerings to include comprehensive solutions for medical ...
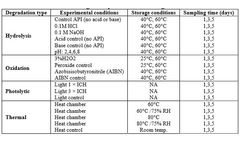
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the launch of its new Force Degradation Services to help pharmaceutical and medical device companies evaluate the stability of their drug candidates and finished products under a variety of stress conditions, ensuring their safety and efficacy throughout their shelf life. Forced ...
BySTEMart
STEMart, a provider of integrated medical device CRO services dedicated to medical device and diagnostic clinical development, now launches Pyrogenicity Testing service for the medical device industry. This new testing follows the biocompatibility guidelines modified for medical devices. Due to the risks associated with medical devices, comprehensive medical device testing throughout the product ...
BySTEMart
Verification is critical for medical device testing, which is why we recommend reading part one if you haven’t already. In part one of this series, we examined the need for medical testing and briefly covered the first few aspects of device verification. In this article, we’ll cover the verification dry run and the formal verification process that makes new medical products a reality. ...
Guided Therapeutics, Inc. (OTCQB: GTHP), the maker of the LuViva™ Advanced Cervical Scan, based on its patented biophotonic technology, announced today its Chinese co-manufacturing partner and distributor for China, Shandong Yaohua Medical Instrument Corporation (SMI), has received notice from the Jinan Medical Device Quality Supervision and Testing Center of the State Food and Drug ...
Attwill Medical Solutions was proud to host U.S. Senator Tammy Baldwin (D-WI) today for a. roundtable discussion regarding the company, as well as a comprehensive factory tour. Attwill Medical. Solutions is one of the largest manufacturers of reagents for COVID-19 and other PCR testing kits, and. has unique freeze-drying technology, which may be utilized for the development of vaccines in a pill. ...
BioSerenity, Inc., a leading global provider of diagnostic solutions in the areas of Sleep Medicine, Neurology, and Cardiology, announced today it has been awarded a group purchasing agreement for PP-SV-268 by Premier as part of its Preferred Partner agreement. The prevalence of sleep disorders, epilepsy and cardiovascular disease pose a significant challenge to the US healthcare system. ...
The National Evaluation System for health Technology Coordinating Center (NESTcc), an initiative of the Medical Device Innovation Consortium (MDIC), announces twelve new Test-Cases which leverage Real-World Evidence (RWE) and address key priorities for medical device stakeholders. These Test-Cases are intended to accelerate NESTcc’s progress and provide proof of concept for NESTcc’s ...
This past November, the U.S. Food and Drug Administration (FDA) announced a Class 1 recall of the Ikaria INOmax DS Drug Delivery System. The device is a drug delivery system used with ventilators to deliver a preset concentration of INOmax therapy gas (nitric oxide for inhalation) for critically ill patients. According to the FDA posting regarding the recall, “Fretting corrosion at the ...
The loss of sterile barrier system integrity may occur as a result of physical properties of the materials and adhesive or cohesive bonds degrading over time and by subsequent dynamic events during shipping and handling. ISO 11607–1:2006, clause 6, states that “the packaging system shall provide physical protection and maintain integrity of the sterile barrier system. The sterile ...
A1.4.1 The purpose of this classification is to establish a consistent terminology system by means of which these ESFD configurations can be classified. It is anticipated that a companion testing standard using this classification system will subsequently be developed. 1. Scope 1.1 This specification provides a characterization of the design and mechanical function of ...