


Cardiac Dimensions - Carillon Mitral Contour System
A completely different and much earlier approach to treating functional mitral regurgitation (FMR) than any other device therapy currently available to patients. The goal: Treat the condition—not just the symptoms. The Carillon System is designed to work with the natural structure and function of the heart to help treat the condition of FMR as well as the related symptoms. We believe the Carillon Therapy is a smarter solution to help patients feel better and live longer lives.
If you have heart failure, you know how this condition affects your quality of life. Simple daily activities like preparing meals, visiting with friends, or even moving from room to room can require major effort and leave you exhausted. An estimated 26 million people suffer from heart failure worldwide, and the number is growing rapidly.
Heart failure is a condition in which the heart is unable to pump enough blood to the body. The heart tries to compensate by growing larger and this increase in size causes the mitral valve to stretch, which in turn leads to the mitral valve leaking and mitral regurgitation. Unfortunately, this further compromises the function of the heart and so the heart failure worsens over time.
Functional mitral regurgitation (FMR) is due to the mitral valve not closing tightly, resulting in blood flowing or leaking backwards in the wrong direction. This further decreases the amount of oxygenated blood that is pumped out to the body and increases the symptoms associated with heart failure and decreases the quality of life of those who have been diagnosed. Approximately 70 percent of those with heart failure have FMR.
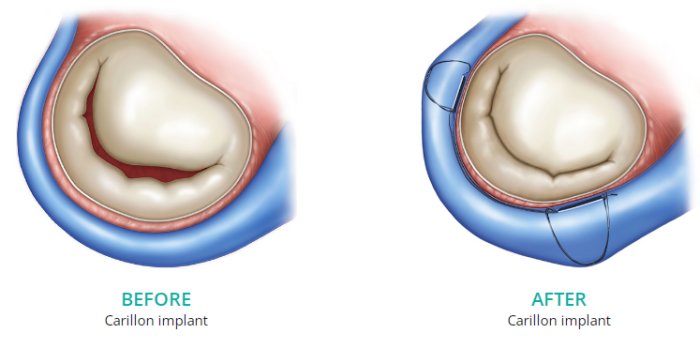
The Carillon Mitral Contour System® is a right-heart transcatheter mitral valve repair (TMVr) device designed to treat the main cause of functional mitral regurgitation (FMR) in patients with MR grades 2+ to 4+.
MR in the context of heart failure is strongly associated with adverse patient outcomes,1 including one-year mortality rates up to 27% if left untreated.2
Designed to reshape the anatomy and improve function of the mitral apparatus from the coronary sinus, the Carillon System reduces regurgitant volume (RV) and induces favorable left ventricular remodeling without compromising the valve3, 4, 5 or future treatment options.6, 7
The distal and proximal anchors of the Carillon device are connected by a shaping ribbon that utilizes the heart’s venous anatomy to cinch the mitral apparatus.
The Carillon device is deployed using familiar catheter techniques and can be recaptured and retrieved prior to release. Additional benefits of this simple right-heart approach include:
- No transseptal puncture
- Short 1-hour average implant time3,4,5
- Ability to treat less sick (MR grade 2+) patients
- Preserves future treatment options such as CRT
- Conscious sedation
- 10 Fr venous access
- No long-term DAPT
- Short user learning curve
To date, four separate trials have evaluated the safety and performance of the Carillon System – AMADEUS, TITAN, TITAN II, and REDUCE FMR. Results have shown that FMR patients treated with the Carillon System experience a mean acute RV reduction of 8 ml and have favorable long-term (3-year) survival.8,9
Results from the latest completed trial, REDUCE FMR, are summarized below.
REDUCE FMR Clinical Trial
The Carillon System is the first TMVr therapy to demonstrate favorable left ventricular remodeling at one year and significant reduction in regurgitant volume in a double-blinded, randomized, sham-controlled trial (REDUCE FMR). The trial met its primary endpoint and results were consistent with the prior TITAN and TITAN II single-arm, multi-center studies.3, 4, 5
Key inclusion criteria
- MR Grade 2+ to 4+
- NYHA Class II–IV
- LVEF ≤50%
Primary endpoint
Change in regurgitant volume at 1 year, as assessed by blinded echo core lab
120 patients in EU and Australia randomized 3:1
- 87 randomized to treatment (Carillon System)
- 33 randomized to sham control (guideline-directed medical therapy)
