

Medicrea
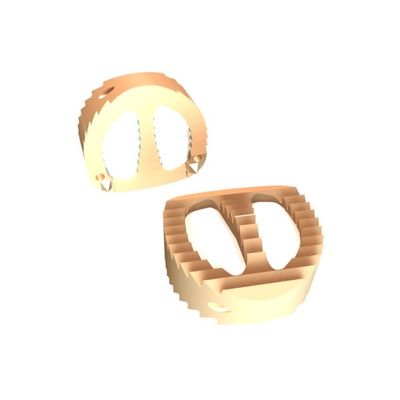
Sterile PEEK Optima - Model IMPIX-C+ -Cervical Interbody Device
FromMedicrea
Enhanced stability: the anatomical dome design with a central wall of IMPIX-C+™ cervical cage provides an excellent stability especially in rotation and offers an efficient fusion solution. Optimum fusion: thanks to a large contact surface between the bone substitute and vertebral plates. Operative time saving: no need for iliac graft swab.
Most popular related searches
bone substitutes
bone substitute
degenerative disc disease
cervical interbody device
disc hernia
cervical pain
cervical spacer
degenerative disc
cervical discectomy
cervical cage
Design:
- 2 windows pre-filled with synthetic bone substitute
- Anti-back out ridging and lower pins to prevent the risk of cage migration or rotation
- Anatomical design to maintain disc height and restore lordosis
- 3 radiographic tantalum markers to control the cage position.
Material:
- PEEK Optima®: radiolucence, bonelike elasticity, biocompatibility
- Bone substitute: > 95% β-TCP.
Complete range:
- 2 sizes: 12.5x14mm 14.5x16mm
- Heights: 4 to 10mm.
Presentation:
- Sterile delivered implant
Patient
60 year-old female with a cervicobrachial neuropathy (disc herniation)
IndicationCervical Degenerative Disc Disease
SurgeryACDFat C6C7 with a PEEK Optima® cervical spacer prefilled with β-TCP substitute, IMPIX-C+™ (not FDA approved) and secured by the compressive staple.
Results
- Significant improvement of Neck Disability Index (NDI), arm and neck pain scores (VAS) at 20 months of follow-up
- Neither pain nor disability was reported by the patient.
