Intellia - Ex Vivo Therapies
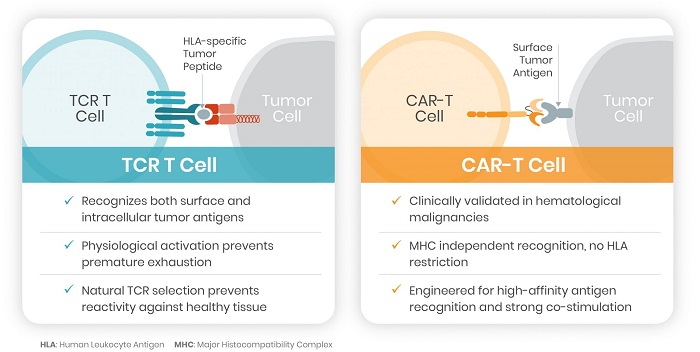
We plan to initiate patient screening for a first-in-human Phase 1 study of NTLA-5001, a WT1-directed TCR T cell therapy for the treatment of AML, by the end of 2021. Our first ex vivo development candidate seeks to treat acute myeloid leukemia (AML) by engineering autologous T cell receptors (TCR) directed towards the Wilms’ Tumor 1 (WT1) antigen, an over-expressed protein that is often associated with AML and other cancers. We have nominated NTLA-5001 as our initial development candidate for AML and expect to enter the clinic in 2021. Intellia’s lead WT1 TCR also has the potential to target WT1-positive solid tumors, such as ovarian cancer, glioblastoma, lung cancer and mesothelioma.
Aside from our proprietary programs, our partnered ex vivo programs with Novartis focus on chimeric antigen receptor T cells (CAR-T cells) and hematopoietic stem cells (HSCs). In March 2020, the U.S. Food and Drug Administration accepted the Investigational New Drug (IND) application submitted by Novartis for a CRISPR/Cas9-based engineered cell therapy for the treatment of sickle cell disease (SCD). OTQ923 is a SCD treatment based on genome editing of HSCs, using CRISPR/Cas9 RNA guides identified through Intellia’s cell therapy research collaboration with Novartis.
We are using CRISPR/Cas9 to selectively and precisely edit the genes of patients’ immune cells, with the goal of improving their performance in treating oncological and immunological diseases. These improved immune cells should more effectively identify and attack cancerous cells upon re-infusion. There is much opportunity to improve the treatment of cancer by using CRISPR with TCR-specific approaches
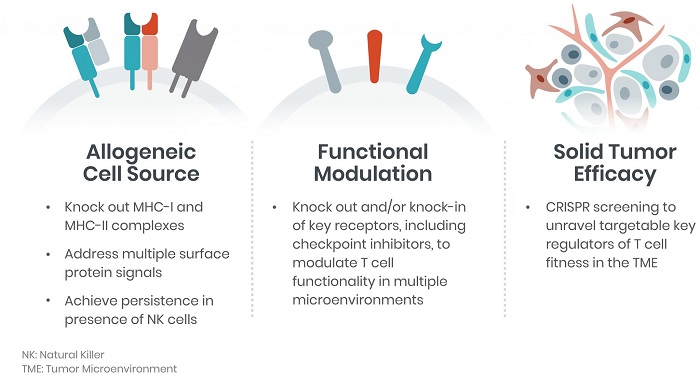
We seek to leverage CRISPR/Cas9 genome editing to create next-generation engineered cells that can treat oncological and immunological diseases for which there are currently limited to no treatment options available. While current cell therapies have improved the standard of care, they are currently restricted to very limited indications. These first-generation cell therapies are also cumbersome and expensive to deliver to patients and may be unavailable to patients who don’t have a healthy enough immune system.
We envision patients completing a single visit to the clinic for administration of an off-the-shelf (i.e., allogeneic) cellular medicine. By applying CRISPR/Cas9 to human cells, we intend to pre-manufacture allogeneic-based cellular therapies, which would be engineered to target tumor-specific markers. This platform would potentially allow these therapies to address many hematological and solid cancers, therefore, representing a truly allogeneic solution for patients.