Model IMGX003 -Latiglutenase Therapy
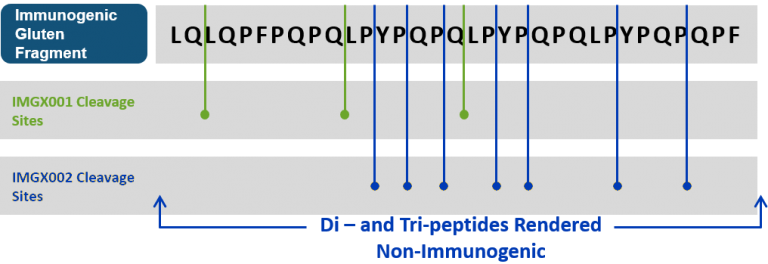
Latiglutenase (IMGX003) is an orally administered mixture of two gluten-specific recombinant proteases that degrades gluten proteins into small physiologically irrelevant fragments, and is to be administered as an adjunct to a gluten-free diet (GFD). As illustrated in the below figure, one protease (IMGX001) is effective at breaking glutamine-leucine bonds and the other protease (IMGX002) targets most proline bonds.
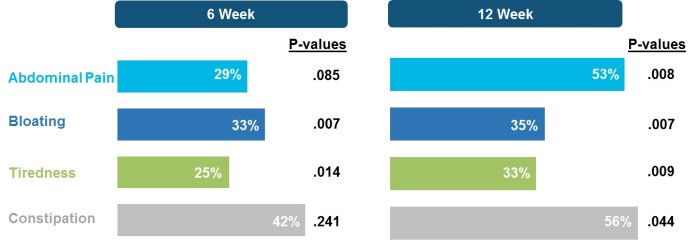
In Phase 2a and 2b clinical trials Latiglutenase has been shown to mitigate gluten-induced intestinal mucosal injury as well reduce the severity and frequency of symptoms in celiac disease patients. Evidence of symptom relief was particularly pronounced for patients who continue to have positive serology to gluten-induced antibodies (seropositive) despite following a GFD as seen in the results shown below (Syage 2017, 2019). These studies also demonstrated that Latiglutenase is well tolerated by CD patients with no discernable difference in adverse event profile for active vs. placebo treated patients.
ImmunogenX is beginning a Phase 2 gluten-challenge study to investigate both mucosal and symptomatic protection in a general population of CD patients. This study will be followed shortly by a Phase 2/3 real-world trial that will focus on symptom improvement in seropositive patients as a prelude to a Phase 3 trial.