

- Home
- Companies
- Taiwan Liposome Company, Ltd. (TLC)
- Products
- BioSeizer - Model TLC399 - Macular ...
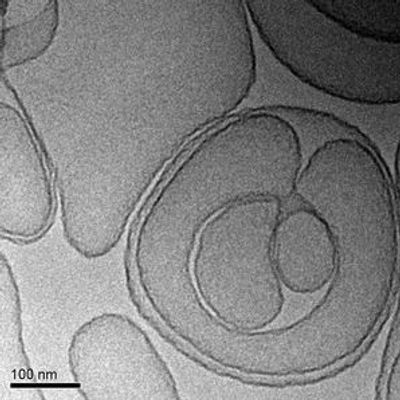
BioSeizer - Model TLC399 -Macular Edema
TLC399 is our proprietary BioSeizer formulation of dexamethasone sodium phosphate (DSP), intended as an intravitreal, or in-eye, injection for the treatment of macular edema due to retinal vein occlusion. TLC399 in preclinical models has been shown to provide therapeutic levels of DSP in the eye for at least six months after a single administration. A Phase I/II safety trial has demonstrated encouraging signs of efficacy in both the reduction of retinal central subfield thickness and improvements in visual acuity. A larger randomized, double-blind, dose finding Phase II trial is underway. We are also evaluating opportunities to develop TLC399 in diabetic macular edema in combination with intravitreal anti-VEGF drugs.
API
Dexamethasone sodium phosphate
DDS Platform
Indication
Macular edema
Development Status
Phase II
- Rapid onset
- Designed to achieve prolonged sustained release duration beyond six months
- Smaller administration needle to reduce risk of conjunctival hemorrhaging and infections
