

- Home
- Companies
- Biogen Technologies S.L.
- Products
- H2O Filters - Model H2OWER1500 - ...
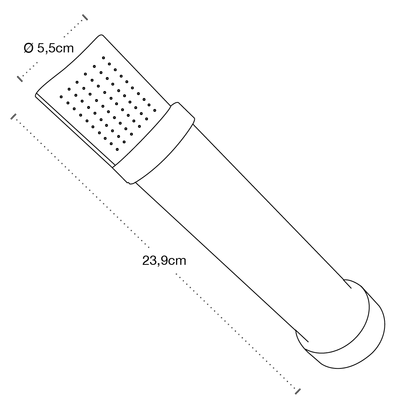
H2O Filters - Model H2OWER1500 -Medical End Point Shower Filter for Pathogen Protection
The H2OWER1500 medical end-point shower filter is designed for use in environments with immunocompromised individuals, providing a critical line of defense against waterborne pathogens. It integrates advanced sterilant membrane technology with a 0.2-micron pore size, delivering sterile-grade filtration without altering water's chemistry. It helps safeguard against nosocomial infections from pathogens like Legionella pneumophila, Pseudomonas, Staphylococcus aureus, and Klebsiella, which are prevalent in both high-risk and low-risk hospital environments. The casing is engineered to resist bacterial proliferation and prevent retrograde contamination from direct contact or splashing. Compatible with systemic water disinfection methods such as chlorination and thermal shock, the filter incorporates traceability via the GS1 Electronic Datamatrix, accessible through a dedicated Biogenfilters app for both iOS and Android platforms.The H2OWER1500 end-point medical shower filter is specially designed to protect immunocompromised patients against all types of pathogens that can be found in water by providing validated sterile grade filtration that does not alter the chemical composition of the water.
It prevents against nosocomial infectious diseases caused by waterborne germs such as Legionella pneumophila, Pseudomona, Staphylococcus aureus, Klebsiela or any other type of pathogen common in high or low risk hospital settings.
Their casing is treated to inhibit bacterial growth and the risk of retrograde contamination either by splashing or direct contact.
Our filters are 100% compatible and resistant to all systemic water pathogen prevention procedures such as chlorination or thermal shock.
- Shelf life: 31 days
- Connection: Direct to ½" flexo
- Membrane: Advanced sterilant of 0.2 um.
- Bacteriostatic treatment: ISO 22196
- Traceability: GS1 Electronic Datamatrix through Biogenfilters APP
