

- Home
- Companies
- Ocugen, Inc.
- Products
- Ocugen - Model OCU400 - Novel Gene ...
Ocugen - Model OCU400 -Novel Gene Therapy Vaccine
OCU400 (AAV-NR2E3) is a novel gene therapy product candidate with the potential to be broadly effective in restoring retinal integrity and function across a range of genetically diverse inherited retinal diseases (“IRDs”). It consists of a functional copy of a nuclear hormone receptor (“NHR”) gene, NR2E3, delivered to target cells in the retina using an adeno-associated viral (“AAV”) vector. As a potent modifier gene, expression of NR2E3 within the retina may help reset retinal homeostasis, potentially stabilizing cells and rescuing photoreceptor degeneration.
In 5 unique mouse models of RP, treatment with the AAV-NR2E3 gene by subretinal injection effectively prevented the further development of multiple genetically diverse IRDs by protecting photoreceptors from further damage after disease onset. The five RP models tested were rd1 (PDE6β associated RP), Rho-/- and RhoP23H (both Rhodopsin associated RP), rd16 (Leber Congenital Amaurosis) and rd7 (Enhanced S-cone Syndrome). The study, published in Nature Gene Therapy, demonstrates the potency of a novel modifier gene therapy to elicit broad-spectrum therapeutic benefits in early and intermediate stages of RP.
OCU400 Preserves Photoreceptors in many mouse models of RP following treatment with OCU400
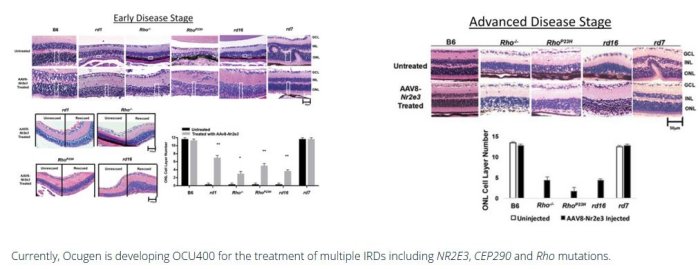
Currently, Ocugen is developing OCU400 for the treatment of multiple IRDs including NR2E3, CEP290 and Rho mutations.
About Inherited Retinal DiseasesRetinitis Pigmentosa (RP) is a group of rare, genetic disorders that involve a breakdown and loss of cells in the retina. According to the National Eye Institute, it is estimated that RP affects approximately 1 in 4,000 people, both in the U.S. and worldwide. RP symptoms often begin in childhood and progress over time. Children often have difficulty getting around in the dark and as their symptoms progress, lose their peripheral (side) vision and eventually experience vision loss and blindness. Because there are more than 150 known gene mutations that cause this disorder, representing only 60% of the RP population, its progression can differ greatly from person to person. The remaining 40% of RP patients cannot be genetically diagnosed, making it difficult to develop individual treatments.
There is currently no approved treatment which slows or stops the progression of multiple forms of RP. Proposed treatments for RP include gene-replacement therapy, retinal implant devices, retinal transplantation, stem cells, vitamin therapy, and other pharmacological treatments. Gene-replacement therapies are promising but are limited to treating just a single mutation and therefore cannot address the multiple mutations implicated by RP. In addition, while gene therapies may provide a new functional gene, they do not necessarily eliminate the underlying genetic defect which may still cause stress and toxic effects. Therefore, the development of gene specific replacement therapy is highly challenging, especially when multiple and unknown genes are involved.
