

- Home
- Companies
- KH Medical
- Products
- Radi Covid-19 Detection Kit
Radi Covid-19 Detection Kit
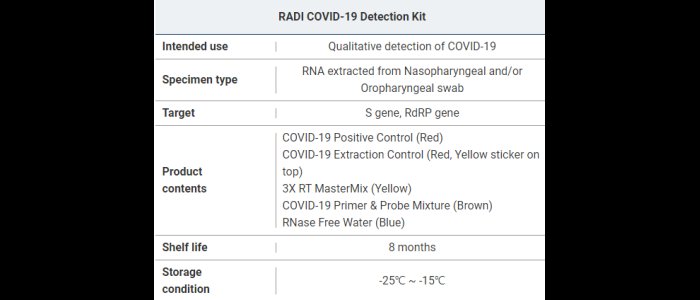
The RADI COVID-19 Detection Kit is intended for the presumptive qualitative detection of specific nucleic acid sequences from the genome of the SARS-CoV-2 S gene and RdRP gene from the upper respiratory specimens such as Nasopharyngeal swab, Oropharyngeal swab.
In order to determine limit of detection (LoD) of RADI COVID-19 Detection kit, a tentative LoD is determined with several diluted concentrations using synthesized RNA by in vitro transcription in 5 replicates. After fixing a tentative LoD is the claimed LoD was determined with 20 replicates of diluted concentrations spanning tentative LoD.
RADI COVID-19 Detection Kit was tested with 63 potential microorganism (including pooled human nasal wash sample).
It was verified that 63 kinds of potential cross-reacting microorganism did not cross-react with any of 63 microorganisms.
RADI COVID-19 Detection Kit was tested with 14 potentially interfering endogenous or exogenous substances in negative clinical matrix with the absence or presence of SARS-CoV-2 (3X LoD).
And it was verified that 14 kinds of potential interfering substances did not have an impact on interference.
The clinical performance of RADI COVID-19 Detection Kit has been evaluated in different countries from different continents.
A total number of 764 samples have been evaluated, and 366 of them were SARS-CoV-2 positive and the rest were negative samples.
