Tsunamed - Model MAGMA -Rapamycin-Eluting Coronary Stent System
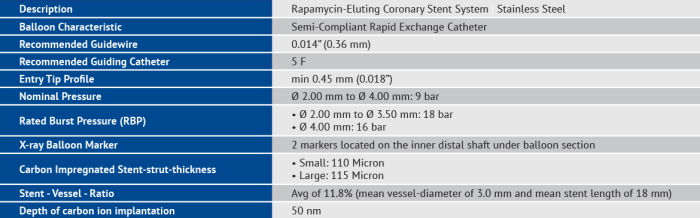
The MAGMA Rapamycin-Eluting Coronary Stent System is the first carbonized stent (Inert Carbon Technology) with a completely biodegradable polymer coating which contains Rapamycin (Rapasorb™) as a highly effective drug for preventing thrombotic and re-stenotic events.
Benefits
- Zero stent thrombosis during the entire implant period
- Polymer: Poly (D, L-Lactide-co-Glycolid) Polylactide 50% Polyglycolid 50%
- Drug: Rapamycin
- Coating Degradation: 6 weeks in-vivo
- Drug load: 2.0μg/mm2
- 250 Patients with over two years follow-up
The Coating
The biodegradable Polymer contains Poly-lactic-co-glycolic acid (PLGA) which will degrade 100% into carbon dioxide and water.
MAGMA does not need any other auxiliary polymer like parylene C.
The controlled polymer degradation and release of Rapamycin is designed to terminate simultaneously and is completed within less than three months. This covers exactly the time where the drug is needed at most and is tailored uniquely to various immune response reactions occurring after stent implantation. This is understood as Rapasorb™ - Technology.