

Metko Medical
- Home
- Companies
- Metko Medical
- Products
- Metko Medical - Model SpO2 - Reusable ...
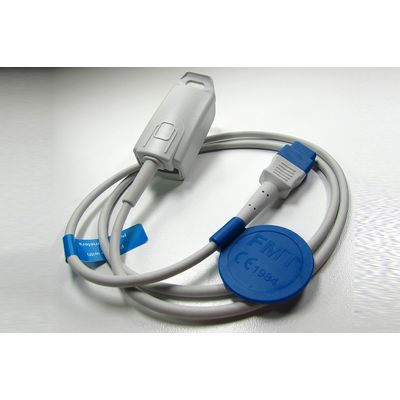
Metko Medical - Model SpO2 -Reusable Sensors for Pulse Oximeters and Patient Monitors
FromMetko Medical
FMT specializes in the manufacturing of reusable SpO2 sensors compatible with a range of pulse oximeters and patient monitoring systems. These sensors utilize precision optoelectronic components to match OEM specifications, ensuring compatibility with devices from brands like BCI, Criticare, GE, and others. FMT offers a variety of sensor types, such as Finger Clip and Neonate Wrap, designed for patient comfort and durability across different age groups. The sensors feature TPU jacketed cables and dual shielding for enhanced signal integrity and longevity. Made from biocompatible materials without the use of latex or PVC, FMT sensors adhere to MDD 93/42/EEC & MDD 2007/47/EC standards and are CE marked, ensuring high safety and minimal bio-compatibility risks.Most popular related searches
MDD 93/42/EEC
pulse oximeter
patient monitoring
patient monitoring system
finger clip
oximeter
oximeter monitoring
patient care
patient size
patient safety
- FMT manufactures reusable SpO2 sensors for different technologies pulse oximeters or the modules built into the patient monitoring systems.
- Selecting precision optoelectronic components that meet the specifications and performance levels of OEM sensors makes FMT sensors fully compatible with most of the pulse oximeters and patient monitors on the market.
- Our entire range of SpO2 sensors is compatible with the following brands or technologies: BCI, Criticare, GE, Mindray/Datascope, Nihon Kohden, Masimo, Nellcor, Nonin, Philips, Spacelabs, and more.
- From neonates to Adults, FMT offers comfortable, durable, and accurate sensors for different patient sizes. Finger Clip, Soft Tip, Multi-Y Style, Neonate Wrap, and Ear Clip type sensor options are available.
- Specially designed, TPU jacketed flexible cable, dual shielded cable construction for signal integrity, bend relief supported cable sections provide extra durability and long service life of FMT SpO2 sensors.
- FMT SpO2 sensors are made of biocompatible materials. No LATEX or PVC is used in the production of reusable SpO2 sensors to maximize patient safety and comfort by minimizing the risk of bio-compatibility problems.
- All FMT SpO2 sensors meet the requirements of the MDD 93/42/EEC & MDD 2007/47/EC and CE marked.
