- Home
- Companies
- Co-Diagnostics, Inc.
- Products
- Logix Smart - SARS-CoV-2 (genes RdRp/e) ...
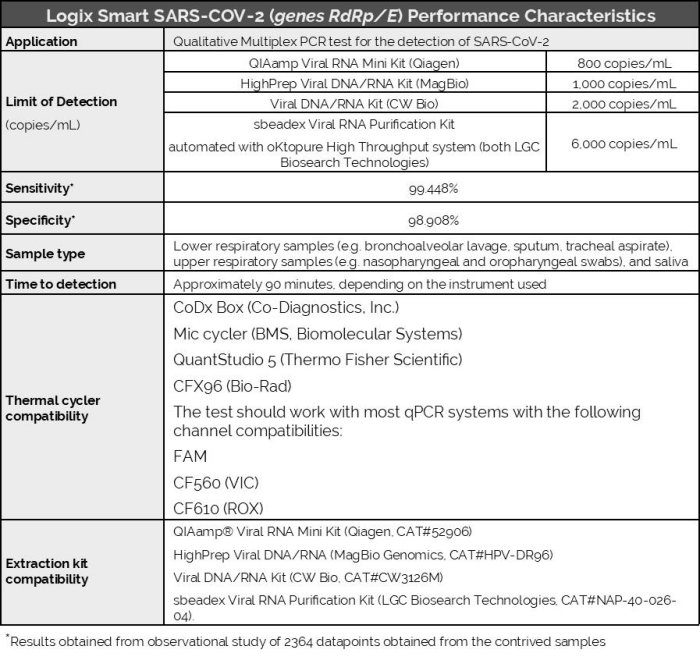
Logix Smart - SARS-CoV-2 (genes RdRp/e) Test Kit
The Logix Smart™ SARS-CoV-2 (genes RdRp/E) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of two genes (gene RdRp and gene E) from the RNA of SARS-CoV-2 coronavirus (COVID-19). The test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in lower respiratory tract fluids (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract fluids (e.g. nasopharyngeal and oropharyngeal swabs), and saliva during the acute phase of detection.
Click here to read the Company’s regulatory bulletin on the ability of our suite of Logix Smart COVID-19 diagnostics to detect all known strains of SARS-CoV-2.
Each Logix Smart SARS-CoV-2 Test kit consists of the following components:
- Ready-to-use Master Mix, complete with RNaseP internal positive control to verify sample quality
- Positive Control (PC), to verify the performance of the master mix
- Nuclease-Free Water as a negative control, to verify master mix is free from contamination
COVID-19 is a contagious respiratory illness caused by the SARS-CoV-2 virus, a relatively new virus in humans. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions have a higher risk of severe illness from COVID-19. Serious outcomes of COVID-19 include hospitalization and death. The SARS-CoV-2 virus can be spread to others not just while one is sick, but even before a person shows signs or symptoms of being sick (e.g., fever, coughing, difficulty breathing, etc.). A full list of symptoms of COVID-19 can be found at the following link:
The Logix Smart SARS-CoV-2 (genes RdRp/E) test is a real-time RT-PCR multiplex test intended for the in vitro qualitative detection of nucleic acid from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), targeting the genes RdRp in the polygene Orf1ab region and gene E of the virus genome, in lower respiratory tract samples (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), upper respiratory tract samples (e.g. nasopharyngeal and oropharyngeal swabs), and saliva from individuals suspected of COVID-19.
The Logix Smart SARS-CoV-2 test is intended for use by trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.