- Home
- Companies
- Focus Laboratories Inc.
- Products
- Focus - Sterility
FOCUS Laboratories can help determine quantities of product needed for adequate sterilization validation.
- Total Organic Carbon (TOC)
Chemical contaminants are also a concern in the manufacture of medical devices. Lubricants, plasticizers, detergents are just some of the undesirable residues that might remain on a device after manufacture and cleaning.
Total Organic Carbon (TOC) is one tool for determining the amount of chemical residue left on a medical device. FOCUS Laboratories can help develop and execute a medical device cleaning validation protocol utilizing TOC.
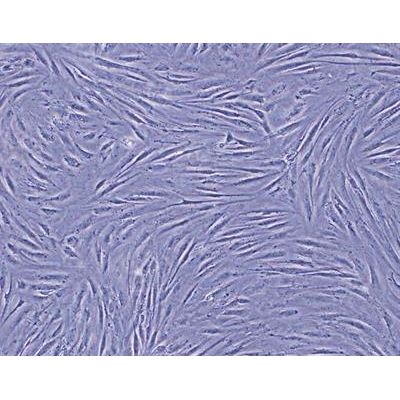
- Cytotoxicity
Medical Devices may have leachable components that can prove to be toxic. USP <87> Biological Reactivity Tests – in vitro provides a method for determining if medical device extracts are potentially harmful. Cytotoxicity testing relies on subjecting a tissue culture to an extract of the device. FOCUS Laboratories can develop and execute a cytotoxicity test for medical device and device components.