- Home
- Applications
- clinical assessment
Refine by
Clinical Assessment Product Applications
11 applications found
Freespira is the only FDA-cleared treatment proven to reduce or eliminate panic attacks and post-traumatic stress disorder (PTSD) symptoms. Unlike medication or talk therapy, in 28 days Freespira addresses the underlying breathing irregularities for individuals suffering from these debilitating anxiety ...
ByFreespira, Inc. based in Kirkland, WASHINGTON (USA)
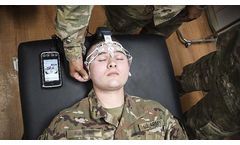
From baseline to return to duty. BrainScope is the only multimodal FDA cleared device that objectively assesses for both brain bleeds & concussions in ...
ByBrainScope Company, Inc. based in Bethesda, MARYLAND (USA)
In order to bring new treatments to patients, we conduct clinical trials to assess the safety and efficacy of our investigational medicines. ...
ByZymeworks Inc based in Vancouver, BRITISH COLUMBIA (CANADA)
The rise of fatty liver disease is an emerging health crisis, resulting in chronic illness for millions of people and escalating healthcare costs. Definitive diagnostic tools for this disease are limited to biopsy and MRI, and are expensive, invasive and time-consuming. To address this crisis, physicians need a better tool to assess and manage ...
BySonic Incytes Medical Corp. based in Vancouver, BRITISH COLUMBIA (CANADA)
The pinnacle of scientific evidence is the Level I study. i-FACTOR Bone Graft is one of a small group of bone grafting technologies that is supported by Level I evidence. i-FACTOR Bone Graft was evaluated in a 319-patient, prospective, randomized, controlled, multi-center clinical trial assessing its safety and efficacy compared to standard-of-care (autograft). ...
ByCerapedics, Inc. based in Westminster, COLORADO (USA)
Range of Motion: Selectively promote or bound each range of motion. ...
ByBioInteractive Technologies based in Surrey, BRITISH COLUMBIA (CANADA)
Save 70% of your time and maximize the probability of success of your clinical study. Avoid common pitfalls by starting your cohort study, patient registry, observational data collection, or clinical trial on Jeeva eClinical cloud platform. Save over 70% of the effort involved in toggling between numerous tools, coordination between multiple channels of engagement between study team and participants, participants use their own mobile device, and study specific data governance. ...
ByJeeva Informatics Solutions Inc. based in Manassas, VIRGINIA (USA)
Stempeucel® can reduce inflammation, which allows the tissues around the fistula tract to ...
ByStempeutics Research Pvt. Ltd. based in Bangalore, INDIA
One of the that cancer can be a serious disease is its ability to spread to other parts of the body beyond the location of the primary tumour. When cancer cells spread to distant lymph nodes, tissues or organs, it is called metastatic ...
ByAscelia Pharma AB based in Malmö, SWEDEN
Phase 3 study assessing the Efficacy and Safety of Stempeucel® in Patients with Moderate to Severe ARDS due to COVID ...
ByStempeutics Research Pvt. Ltd. based in Bangalore, INDIA
Increase clinical translation with Organ-Chips for ADME-Tox assessments. Accurate ADME-Tox profiling of preclinical drug candidates remains a challenge. ...
ByEmulate based in Boston, MASSACHUSETTS (USA)