- Home
- Companies
- Samyang Corporation
- Applications
- Trilite - Ion Exchange Resin for ...

Trilite - Ion Exchange Resin for Pharmaceutical & Biotechnology - Chemical & Pharmaceuticals - Pharmaceuticals
TRILITE® ion exchange resins and synthetic adsorbents can be used in various separation and purification processes for pharmaceuticals and food raw materials. Some representative examples are as follows.
Separation of Non-Electrolytes and Electrolytes
A process of removing electrolytes (various cationic and anionic impurities) from
non-electrolytes (such as starch sugars) using cation and anion exchange resins. Typical examples include starch sugar refining, sugar refining, sorbitol refining, glycerin refining, and gelatin refining processes.
Separation of Amphoteric Electrolytes
There are processes for separating amino acids and antibiotics that dissociate into cations or anions at specific pH levels by utilizing ion exchange to separate them. Lysine purification/refinement and nucleic acid separation/refinement are representative examples In addition, a separation can be carried out at the isoelectric point (pH) by using Ion-Exclusion Chromatography and separation and purification of arginine is a representative example. For amino acids with nonpolar groups, separation can be carried out using synthetic adsorbents at the isoelectric point, and the separation/purification of L-isoleucine is a representative example.
Separation based on differences in molecular weight or hydrophilicity/hydrophobicity
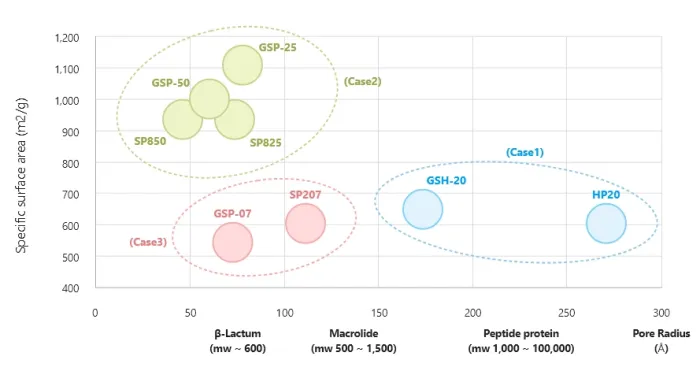
Separation can be achieved using synthetic adsorbents with various pore sizes and surface areas based on the principle of molecular sieves, or by using Van der Waals forces for non-polar substances. The separation of cephalosporin C is a representative example. Synthetic Adsorbents are also widely used in various applications such as separation, purification, and decolorization of pharmaceuticals and food products.
Electrolytes
Substances that dissolve in a solvent such as water and dissociate into ions, allowing electric current to flow. Generally, substances that can be exchanged with ion exchange resins are collectively referred to as electrolytes.