Refine by
Medical Devices At The American Medical Device Articles & Analysis: This-Year
14 news found
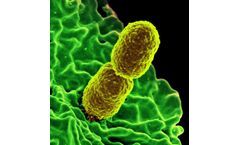
Klebsiella is described by the Centers for Disease Control and Prevention (CDC) as a type of gram-negative bacteria that can cause different types of healthcare-associated infections (HAIs). These include pneumonia, bloodstream infections, wound or surgical site infections, and meningitis. Klebsiella are normally found in the human intestines (where they do not cause disease), and also in human ...
Many medical devices are manufactured through plastic injection moulding, which allows for precise and top-quality components in large-scale production and better aligns with ISO standards. In general, injection moulding for medical devices is conducted in an ISO 8 clean room environment to ensure all products adhere to the strictest standards of sterility and safety.This article covers how ...
Watertown, MA - June 29, 2025 - Biopharma PEG, a leading innovator in high-quality polyethylene glycol (PEG) derivatives, today announced it has successfully obtained Drug Master File (DMF) approval from the U.S. Food and Drug Administration (FDA) for its self-developed HZ-PEG-HZ (1K) product. The assigned DMF number is 041864. This significant achievement underscores Biopharma PEG's robust ...
I.R. Tubes Pvt Ltd, a leading supplier of specialty chemicals in India, today announced the expansion of its product line to better serve the rising demand in pharmaceutical, medical device, and industrial manufacturing sectors. The company’s latest initiatives are aligned with India’s "Make in India" vision, aiming to deliver advanced chemical solutions that meet global regulatory and ...
On April 22, MedSource Labs welcomed Andrea Mitchell and her NBC film crew to our office to meet with our leadership team and discuss tariffs and the business strategies we are deploying to mitigate impacts to our customers and global partners. As shown in the segment that aired on April 25 on NBC Nightly News, tariffs have significantly affected our mission to manufacture and deliver ...
Changsha, China – 18 March 2025 – Hunan Huateng Pharmaceutical Co., Ltd., a global leader in PEG derivatives and CDMO services for APIs and intermediates, is pleased to announce its participation in CPHI Japan 2025. The company will exhibit its comprehensive product lines and cutting-edge capabilities at Booth No. 4X-06, located at Tokyo Big Sight, Tokyo, Japan, from April 9th to ...
Obtaining the Marketing Authorisation Application (MAA) is a key step in the commercialization of drugs in the EU, and also an important help to promote the global market expansion of pharmaceutical companies. The MAA is complicated by the need to consider both European economic integration and the independence of individual member states. As a specialist in regulatory matters, Proregulations has ...
In this free webinar, learn about the importance of early glycan screening for ensuring biologic quality, and how automation reduces time-to-insight and improves workflow efficiency. The featured speakers will share real-world applications and results from glycan screening success. Attendees will learn how to integrate automated glycan profiling into cell line development (CLD) labs. Early ...
U.S. medical device regulations require manufacturers of most class II and a small number of class I devices to file a 510(k) unless they qualify for an exemption. 510(k) is a premarket technical document submitted by the manufacturer to the FDA before the medical device product enters the US market to prove that the product has the same safety and effectiveness in terms of intended use, design, ...
Last year, during Black History Month, our team at MedSource Labs took a closer look at the world of medical illustration and the ongoing efforts to address the lack of diversity in the field. We were inspired by the work of medical illustrators who are breaking barriers and ensuring that healthcare visuals represent the full spectrum of patients they serve. To see video, please click here What ...
The FDA regulates cosmetics and their ingredients on the U.S. cosmetics market. Strict adherence to FDA regulations and standards is essential for the smooth entry and successful operation of cosmetic products on the US market. At Proregulations, a company that specializes in assisting with regulatory compliance, we offer U.S. Cosmetics Registration services designed to ensure that cosmetics meet ...
An Abbreviated New Drug Application (ANDA) is an application submitted to the U.S. FDA to demonstrate that a generic drug is equivalent to a previously approved Reference Listed Drug (RLD) in terms of safety, efficacy, and quality. The ANDA contains information used for the review and approval of a generic drug product, and does not typically require preclinical and clinical trial data, but ...
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announces the expansion of its medical device testing capabilities and introduces Balloon Catheter Testing Services for the development of safe and effective medical devices. These reliable testing solutions will meet the evolving needs of the medical device industry and help manufacturers ...
BySTEMart
With years of experience in the pharmaceutical and life science sectors, CD Bioparticles announces the launch of its new line of Endotoxin Free Silver Nanoparticles for biomedical research and bioassay development. These highly purified nanoparticles offer exceptional performance and reliability in various research and commercial applications, such as drug delivery, nanosensors, photothermal ...