- Home
- Companies
- Thermo Fisher Scientific
- Products
- Thermo Scientific - Model MAM 2.0 ...
Thermo Scientific - Model MAM 2.0 Workflow - Biopharmaceutical Analytical Testing
MAM has been broadly embraced by the biotherapeutic industry and regulatory leaders in recent years. Major biopharmaceutical companies are making significant investments to establish a MAM workflow to future proof the development and manufacturing of their biotherapeutics. Historically, the implementation of MAM has not been easy or fast due to the absence of a complete commercial MAM solution. Scientists have had to piece together multiple hardware and software components originally designed for different purposes. Such MAM solutions often require expert MS users and are not without challenges when deployed in process development and QC environments. Being the leading biotherapeutic solution provider and a trusted partner, Thermo Fisher Scientific has been working closely with industry leaders to develop a seamless, fit-for-purpose MAM solution. Significant improvement has been achieved with the introduction of Thermo Scientific MAM 2.0 since the initial HR MAM launch in 2019.
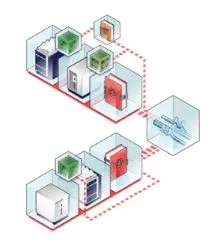
Build your process knowledge within a compliance-ready, enterprise MAM data system that connects instruments, functions, departments and sites throughout development and manufacturing, enabling seamless data and knowledge sharing.
Simplify your routine operations without compromising data quality on Thermo Scientific Orbitrap Exploris MX mass detector, built on the same platform as Thermo Scientific Orbitrap Exploris 240 mass spectrometer. Eliminate lengthy method development in QC with direct method transfer within compliance-ready Chromeleon CDS software.
The HR Multi-Attribute Method (HR MAM) is the first complete commercial MAM solution that delivers comprehensive characterization and monitoring of biotherapeutic products. The HR MAM workflow employs all the necessary components for a successful peptide mapping-based biopharmaceutical characterization workflow. The workflow includes:
- System suitability test standard and high-quality solvents.
- Industry-leading, high-resolution Thermo Scientific Q Exactive Plus Mass Spectrometer coupled to a state-of-the-art Thermo Scientific Vanquish Horizon UHPLC system and Thermo Scientific Accucore Vanquish C18+ HPLC column.
- Thermo Scientific BioPharma Finder Mass Informatics Platform for Biopharmaceutical Characterization and compliance ready Chromeleon CDS Software for data acquisition, attribute characterization, and new peak detection (NPD).
- Fast decision making based on high confidence information of multiple product quality attributes (PQAs) from development through QC, ensured by industry proven Orbitrap technology and Thermo Scientific ChromeleonChromatography Data System (CDS) as GMP supporting data acquisition and processing software.
- Seamless knowledge sharing and method transfer across instruments, functions, departments, and sites throughout the whole organization to accelerate development, enabled by a compliance-ready enterprise data system.
- Maximum productivity through a dedicated global support team of Thermo Fisher MAM experts, providing application specific training and service.