
- Home
- Companies
- Datar Cancer Genetics
- Products
- Trublood - Model 360 - Blood-Based ...
Trublood - Model 360 - Blood-Based Diagnosis Technology
Trublood is an advanced non-invasive blood test (which provides diagnostically relevant information about cancers via a safe, simple and quick blood draw). Trublood® evaluates blood sample to detect ‘Circulating Tumor Cells’ (CTCs), which are cancer cells that escape from the tumor and enter the blood stream. Trublood® enriches these CTCs from the blood sample and confirms their identity via a process called ‘Immunocytochemistry’ (ICC). A positive CTC finding is indicative of the presence of cancer whereas when CTCs are undetectable, the patient may not have a cancer.
- Patients with suspicious findings such as externally visible lumps on the body or in whom medical imaging (endoscopy, CT, MRI or USG) detected an abnormal growth (‘tumor’) maybe suspected of cancer. Depending on where the suspicious finding is located in the body, about 50% - 80% of these tumors may actually be non-cancerous (benign) and without significant health risks.
- However, confirming this (cancer v/s benign) requires a sample of the tumor tissue to be tested by ‘histopathological examination’ (HPE). The tumor tissue sample is obtained either by a surgery or via a biopsy; these ‘invasive procedures’ need an incision (cut) to be made on the skin using a scalpel to take a sample of the suspicious tissue from within the body.
- Tissue sampling is associated with risks such as pain, soreness, bleeding and infection. For some organs (e.g., lungs or brain), these risks may be even greater. Sometimes, tissue sampling may not be possible because of these risks. In some cases, insufficient tissue sample may lead to a dilemma due to inconclusive findings. In the cases where these problems are encountered, a repeat tissue sampling procedure may be considered but may not always be possible.
Identifying patients with a higher likelihood of having cancer and prioritizing them for follow-up (‘triaging’), which can reduce the need for (and risks of) invasive procedures in patients who are more likely to have non-cancerous conditions.
A safer and robust alternative to tissue sampling-based method, which can be relied upon to provide meaningful information to support a diagnosis in cases where there are challenges to tissue sample - based diagnosis.
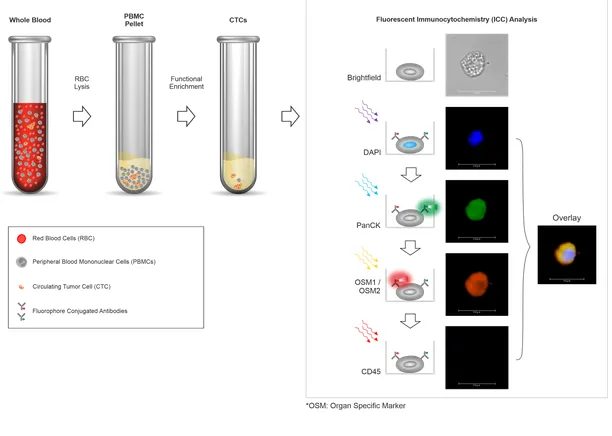
Trublood® detects CTCs, which are known to be present (and detected ubiquitously) in most types of cancers called solid tumors. Several prior clinical studies, including DCG’s own acclaimed studies have shown that CTCs are detectable even in very early Stage 0 (in situ cancer) as well as Stage I and Stage II cancers. CTCs are detected across all subtypes, in men and women and in treated patients as well as in those who have not received any anti-cancer treatments (‘therapy naïve’). CTCs do not exist in individuals without cancer. So CTCs are undetectable in blood of patients with noncancerous (benign) tumors. Owing to these properties, CTCs have high accuracy for cancer detection.
