- Home
- Companies
- InMed Pharmaceuticals Inc.
- Products
- InMed - Model INM-755 - Cannabinol ...
InMed - Model INM-755 - Cannabinol Topical Cream for the Treatment of Epidermolysis Bullosa
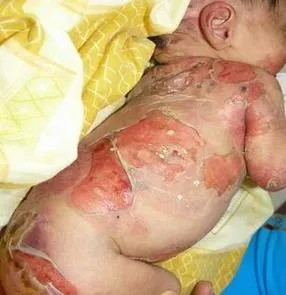
INM-755 is a cannabinol topical cream under development for the treatment of epidermolysis bullosa. INM-755 cream for EB is the first, and currently the only, cannabinol formulation being tested in clinical trials as a therapeutic product.
InMed has commenced a Phase 2 clinical trial of INM-755 (cannabinol) cream in Epidermolysis Bullosa (“EB”). This study will be taking place at 13 sites across eight countries including Austria, Germany, Greece, France, Italy, Israel, Serbia and Spain. Five clinical sites have been fully activated. Enrollment and patient treatment began in December 2021 and are expected to complete during the calendar year 2022.
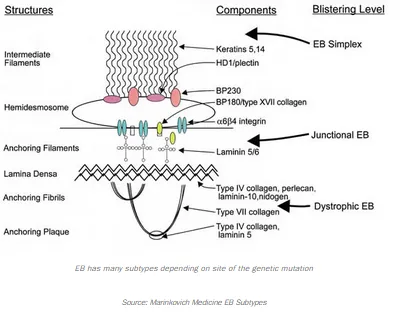
The Phase 2 study, 755-201-EB, is designed to enroll up to 20 patients. All four subtypes of inherited EB, being EB Simplex, Dystrophic EB, Junctional EB, and Kindler Syndrome, are eligible for this study. InMed will evaluate the safety of INM-755 (cannabinol) cream and its preliminary efficacy in treating symptoms and healing wounds over a 28-day period. The study will use a within-patient, double-blind design whereby matched index areas will be randomized to INM-755 (cannabinol) cream or vehicle cream as a control. To learn more about this EB study, view the detailed study description on the National Institutes of Health (NIH) clinicaltrials.gov website.
Preclinical studies show CBN potential in managing symptoms of EB as well as improving skin integrity in a subset of EB patients
InMed completed extensive safety pharmacology and toxicology studies of CBN and INM-755 cream that demonstrated promising results and supported advancing the compound into clinical trials.
In preclinical pharmacology studies, CBN demonstrated activity in reducing markers of inflammation and pain. It also upregulated expression of a type of keratin (keratin 15, or K15), which might lead to improved skin integrity and reduced blister formation in EB simplex (EBS) patients with mutations of another keratin (keratin 14, or K14). Its anti-inflammatory activity may be beneficial in healing chronic wounds where healing has been prevented by prolonged inflammation.
CBN demonstrates an excellent safety profile
InMed conducted several preclinical safety pharmacology and toxicology studies using CBN at very high doses that achieved systemic exposure (blood levels) hundreds of times higher than what is expected to occur with topical dosing in humans. No adverse events were seen on central nervous system (CNS) function in a rigorous and extensive evaluation of CNS effects; 108 aspects of behavior posture, gait, and movement were assessed. In that study, the blood levels were more than 10,000 times what is expected to occur with topical dosing in humans. No adverse effects were observed in preclinical studies where the drug was applied either as a cream (for local effects) or injected under the skin (for systemic effects) daily for 28 days, even at the highest doses.
Results from two Phase 1 clinical studies of INM-755 cream in healthy volunteers treated for 14 days indicated that INM-755 cream was safe and well-tolerated on intact skin as well as open epidermal wounds, caused no systemic or serious adverse effects, and there were no subject withdrawals due to adverse events.