
- Home
- Companies
- Kuros Biosciences A.G.
- Products
- Model KUR-113 - Drug-Biologic ...
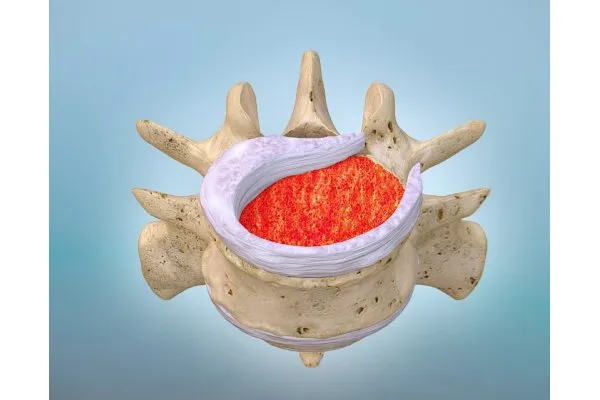
Model KUR-113 - Drug-Biologic Combination for Spinal Fusion
Fibrin-PTH (KUR-113) is the latest candidate in our pipeline – and the first ever investigational drug-biologic candidate to be evaluated for spinal fusion. It’s based on proprietary controlled-release technology that combines the well-established mechanism of the bone growth factor parathyroid hormone (PTH) with the natural healing matrix, known as fibrin.* Once implanted, Fibrin-PTH promotes spinal fusion by increasing the number and lifespan of bone-forming (osteogenic) cells in the fusion space. Fibrin-PTH is also the first investigational drug-biologic to be compatible with narrow gauge cannulas for truly non-invasive surgical procedures – with the potential to be a true game changer once commercially available.
Fibrin-PTH has featured in two separate Phase 2 randomized, controlled, open-label, dose-blinded studies – each featuring 200 patients.1,2
Tibial plateau fractures: the statistical non-inferiority of Fibrin-PTH to autograft was demonstrated based on the proportion of patients who achieved radiological fracture union at 16 weeks, post-graft. Thus, the study achieved its primary efficacy endpoint.
Tibial shaft fractures: the healing rate at six months post-surgery for intend-to-treat patients was 76%, 80% & 69% for patients receiving the low, medium & high doses of Fibrin-PTH respectively – versus just 65% for those receiving Standard of Care.
