
- Home
- Companies
- Cognoptix Inc.
- Products
- Cognoptix - SAPPHIRE II System
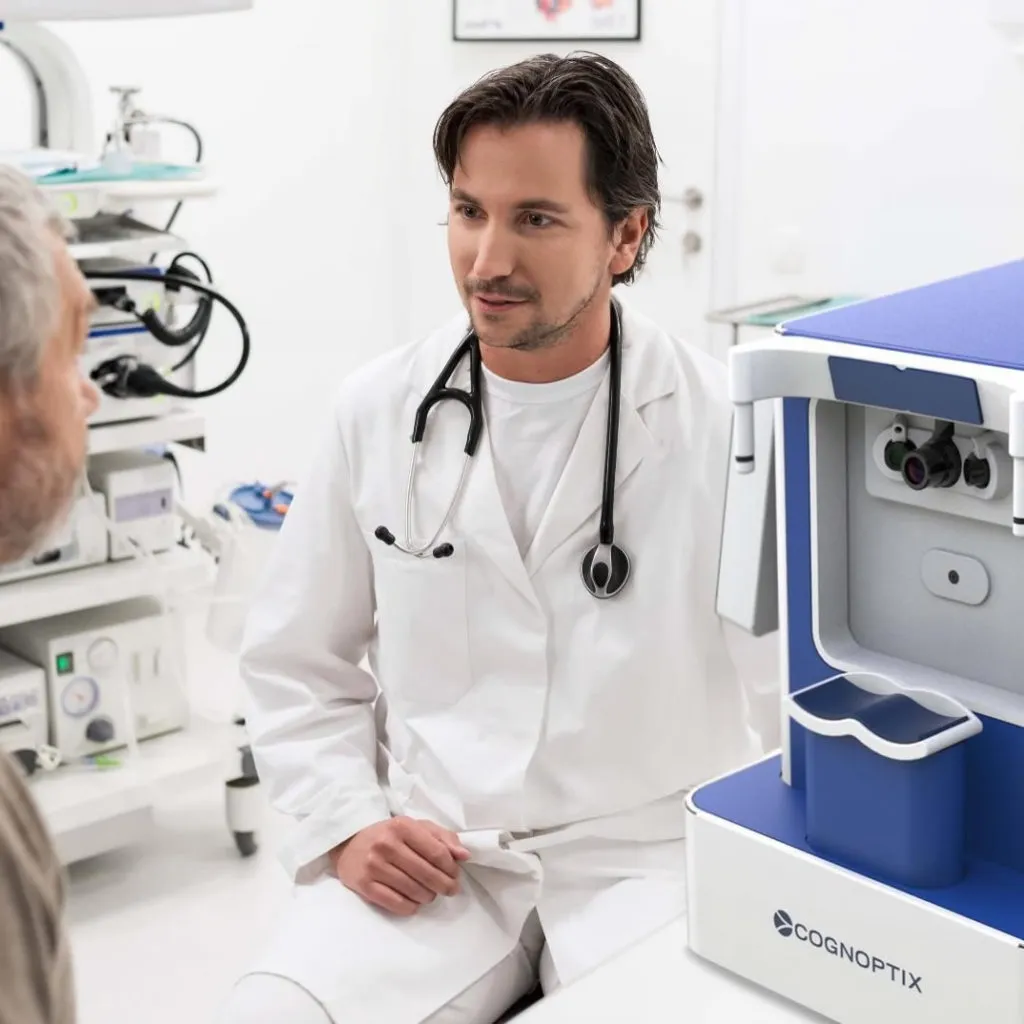
Cognoptix - SAPPHIRE II System
Cognoptix’ SAPPHIRE platform is a compact, easy to use, clinical device for in situ patient examination in the comfort of a doctor’s office. The combined platform is currently available in the United States for investigational use only and is intended for clinical evaluation and measurement of beta-amyloid aggregation in anatomically defined regions of the anterior segment of the eye.
The results of a multicenter clinical trial of its SAPPHIRE II eye test, designed to identify Alzheimer’s Disease (AD) patients via a beta-amyloid signature in their eyes, has been published in the peer-reviewed Journal of Alzheimer’s Disease & Other Dementias (AJA).
By detecting a specific fluorescent signature of ligand-marked beta-amyloid in the supranucleus region of the human lens, SAPPHIRE II achieved a sensitivity of 85% and a specificity of 95% in differentiating 20 patients who were clinically diagnosed with probable AD from a group of age-matched, healthy volunteers. In addition, the SAPPHIRE II test showed excellent correlation to PET (positron emission tomography) amyloid brain imaging, the current gold standard for detecting beta-amyloid in the brain.
