

PsiOxus - Model T-SIGn - Viral Vector Platform Technology
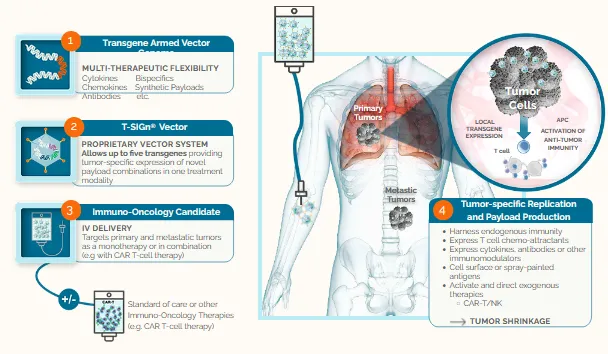
Our clinically validated T-SIGn® viral vector platform delivers multiple transgene payloads that re-program the tumor microenvironment of both primary and metastatic tumors. Our platform can integrate rationally designed synthetic agents to unleash the power of the immune system, including genetically engineered cell therapies.
The platform enables intravenous (IV) delivery of multi-armed genetic payloads to tumors and metastases. The viral vector replicates only in cancer cells and the replicating virus can persist in tumors for many weeks. The breadth of therapeutic opportunities for combination vectors include cytokines, chemokines, antibodies, bispecific therapeutics, and other novel synthetic payloads.
Our technology has been mechanistically designed to target carcinomas that account for 80-90% of all cancer cases and is highly-leverageable across a wide range of cancer types.
Marrying our mastery of vector design with our deep expertise in immunology and biology of the tumor microenvironment opens a multitude of potential opportunities for innovative combination therapeutic approaches for treating cancer.
In addition to conventional biologic molecules, such as antibodies, antibody fragments, bi-specifics, cytokines, chemokines and other immunomodulators, we are also exploring novel synthetic payload designs as we build an exciting and powerful pipeline of new T-SIGn® candidates, both independently and through partnership.
The clinically demonstrated capability of our vectors to selectively produce their transgene payloads in tumor cells following intravenous infusion has enabled us to break free from the constraints hampering traditional oncolytic viruses, potentially enabling the treatment of patients with both primary and metastatic disease.
We are experts at designing vector constructs, and the payload opportunities are endless. Our T-SIGn® tumor re-programming technology extends beyond traditional oncolytic viruses and we are continuing to explore other novel synthetic payloads and ways of combining more agents to develop a powerful pipeline of new T-SIGn® opportunities. These can include antibody fragments, bispecifics, engagers, and other immune-modulators, rationally designed to target specific tumor indications.
There remain numerous obstacles to successfully treating solid tumors, including:
- Poor immune cell infiltration into, and survival within, these tumors
- Immunosuppressive properties of the tumor microenvironment (TME)
- Lack of tumor selectivity of target antigens which leads to issues with systemic toxicity
Reprogramming the TME using T-SIGn® viral vectors could enable a variety of CAR-T and other cell therapies to overcome such limitations and lead to successful treatment for a variety of different solid tumors.
Preclinical human tumor xenograft studies have demonstrated that T-SIGn viral vectors (expressing transgenes designed to reprogram the TME and drive immune cell recruitment and activation) potently synergize with CAR T-cells of different specificities to clear both primary and metastatic tumors:
- Gene expression profiling demonstrated T-SIGn-mediated TME reprogramming and enhanced T-cell activation, as well as recruitment of innate immune cells into the tumors
- Transgene payloads were shown to drive the efficacy of T-SIGn/CAR-T combination
- Metastases in lungs were also synergistically cleared in this system
T-SIGn viral vectors can be exquisitely designed to specifically synergize with different engineered cellular therapies, including the expression of target antigens and ligands that enhance activation, survival and functional properties of the cells.
Click here for further information on our cell therapy collaboration >
Cell-based therapies, particularly those based on T-cells expressing a chimeric antigen receptor (CAR T-cells), have proven to be highly effective for patients with hematological cancers.
