
AQA Company, Inc.
- Home
- Companies
- AQA Company, Inc.
- Software
- IMSXpress - Version ISO 14971 - Medical ...
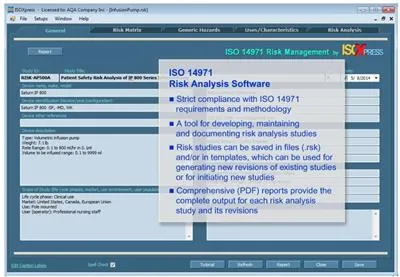
IMSXpress - Model ISO 14971 - Medical Device Risk Management and Hazard Analysis Software
IMSXpress 14971 Medical Device Risk Management software is a Windows application for implementing Risk Analysis, Risk Evaluation, and Risk Control in strict compliance with the ISO 14971:2012 standard. Risk analysis, risk evaluation, and risk control methodologies strictly follow requirements of ISO 14971 and all recommendations included in ISO 14971 Annex B through E. Reports generated by IMSXpress comply with ISO 14971 requirements for Risk Management File (Clause 3.5) and provide most of the content required for that file.
Most popular related searches
There is a comprehensive tutorial included with the software. It explains all the stages (steps) of developing a risk study, and the tools and techniques available in the software for implementing the study.
The tutorial consists of several slide shows with screen shots of the software and an instructional narrative. The slide show format is the easiest and most efficient way to learn how to develop the risk study and how to use the software.
The following slide shows are available in the tutorial:
- Risk Study Overview
- Risk Matrix
- Generic Hazards
- Uses and Safety Characteristics
- Risk Analysis
- Risk Control
- Reports
Risk studies are saved in files and/or templates
Risk management studies are saved in into .rsk files -- just as documents are saved as .doc/.docx files in MS Word.
Risk studies can also be saved as templates. These can be blank studies with pre-defined risk matrices, generic hazards, safety characteristics, etc.IMSXpress ships with a template that includes generic hazards and generic safety characteristics.
Customizable PDF Reports
PDF Reports generated by IMSXpress comply with ISO 14971 requirements for Risk Management File (Clause 3.5) and provide most of the content required for that file. There is even an approval signature page, so that the report is ready for publishing as a controlled document and/or for submitting the study to your regulatory agency. A full report includes the following sections:
- Title Page
- Risk Matrix
- Table of Contents
- General Information
- Approvals (signature page)
- Risk Matrix
- Risk Study Summary Report
- Risk Study Detailed Report
- Appendix 1 - Generic Hazards
- Appendix 2 - Considered Uses and Safety Characteristics
The reports are highly customizable:
- There is an integrated editor to help you compose a header including company logo, background image, and additional lines of formatted text
- Every caption in the report can be edited, allowing you to customize the report template, and even translate the template into any language
- You can choose which sections to include in the report
