- Home
- Applications
- usa colorado
- clinic celiacshield trial
Show results for
Refine by
Clinic Celiacshield Trial Product Applications In Usa Colorado
2 applications found
The pinnacle of scientific evidence is the Level I study. i-FACTOR Bone Graft is one of a small group of bone grafting technologies that is supported by Level I evidence. i-FACTOR Bone Graft was evaluated in a 319-patient, prospective, randomized, controlled, multi-center clinical trial assessing its safety and efficacy compared to standard-of-care (autograft). Patients underwent anterior cervical discectomy and fusion (ACDF) and received either i-FACTOR Bone Graft or local autograft in a cortical allograft ring ...
ByCerapedics, Inc. based in Westminster, COLORADO (USA)
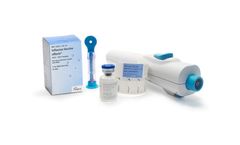
The first vaccine in the US labeled for needle-free delivery. Afluria® and Afluria QIV Influenza Vaccines are FDA Approved for use with the PharmaJet Stratis® Jet Injector Needle-free System. The Stratis Needle-free Injector is the first needle-free jet injector that is FDA approved for delivery of an influenza vaccine. A comprehensive clinical trial demonstrated that needle-free flu vaccine delivery with Afluria® is comparable to administration using a needle and syringe for adults aged 18-64. ...
ByPharmaJet based in Golden, COLORADO (USA)