- Home
- Applications
- USA
- monoclonal secondary antibody
Show results for
Refine by
Monoclonal Secondary Antibody Product Applications In Usa
4 applications found
Premium
Discover a high-throughput flow imaging microscopy platform for characterizing API aggregates and other particulates in your protein, monoclonal antibody, or antibody-drug conjugate ...
ByYokogawa Fluid Imaging Technologies, Inc. based in Scarborough, MAINE (USA)
NorthStar Medical Radioisotopes is poised to become the first-in-class supply chain for the future of therapeutic radiopharmaceuticals. Check back for progress on our non-carrier-added (n.c.a) Ac-225 and Cu-67 development ...
ByNorthStar Medical Radioisotopes, LLC based in Beloit, WISCONSIN (USA)
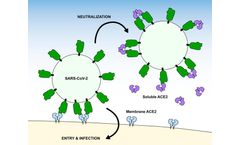
As the COVID-19 pandemic continues, the SARS-CoV-2 virus is likely to become endemic with emerging novel variants, some of them seasonal. Updates to the various vaccines will be required, as will a range of anti-virals with a variety of mechanisms of action, for use in early infection and in hospitalizated patients, much like oseltamivir (Tamiflu) is used in certain populations for seasonal ...
ByCyrus Biotechnology Inc. based in Seattle, WASHINGTON (USA)
The manufacture of biopharmaceutical agents such as therapeutic monoclonal antibodies, vaccines and cell-based therapies like CAR-T cells require the highest quality control against the contamination of adventitious agents. These pathogens can enter the manufacturing process at multiple stages and impact yield and safety. Current methods for detection of adventitious agents are expensive, lack sensitivity and have long turn around times to results. Moreover, many of the current adventitious agent testing methods ...
ByAccuGenomics, Inc. based in Wilmington, NORTH CAROLINA (USA)