Vaccine Handling And Storage Articles & Analysis
20 articles found
We built a more than 700,000-cubic-foot vaccine storage solution for a global manufacturer, during COVID-19 lockdown, on time and on budget. ...
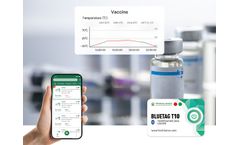
The optimal storage environment temperature for most vaccines is 2-8℃. It is necessary not only to control the temperature stability but also to monitor the temperature. The 2-8℃ Vaccine Bluetooth Temperature Sensor can help managers view the storage environment temperature data directly on the APP without opening the incubator, thereby improving the efficiency of cold chain temperature ...
Glycerin, a versatile and widely used compound, finds its application in various industries, including pharmaceuticals. By understanding the multifaceted applications of glycerin in vaccine formulation, storage, and delivery, pharmaceutical manufacturers can optimize their vaccine production processes to ensure superior quality and effectiveness. Among the numerous components and additives ...
Due to the particularity of vaccines, vaccines need to be kept in a constant and suitable temperature environment during transportation and storage, and different vaccines require different environmental temperatures. Therefore, vaccines often need to be processed in the cold chain, and Temperature Humidity Recorders with quality certification are required to monitor and record the temperature of ...
Vaccine Freezers are required for the safe storage of vaccines that must be kept at extremely low temperatures. Without sufficient storage, a vaccine is likely to form deviations, drastically impacting the vaccines efficiency. At Froilabo we work to support the medical and pharmaceutical industry with the equipment they require to ensure safe storage solutions. Vaccine storage has been in high ...
COVID -19 Vaccine Overview The development of a COVID-19 vaccine by drug manufacturers has been welcomed across the globe. A key concern, however, is the safe distribution of the vaccines worldwide. This may cause some challenges for certain vaccines. Certain vaccines must be kept at an extremely cold temperature of -70°C (-94F), much lower than the average temperature capabilities of an ...
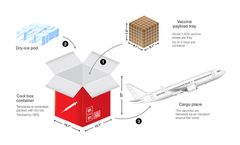
Transportation Specifically, let’s look at the COVID-19 vaccine storage and handling file to see what they are required to know.ll of the ult and freezer storage units that will be storing the COVID-19 vaccine must be set up to stabilize temperatures at the recommended temperature range specified by the ...
ByUbiBot
[ii] Ensuring flu vaccines remain effective through proper storage not only helps guarantee that those who are vaccinated are protected but also makes people more likely to continue opting to receive vaccines. ...
Reliable medical refrigeration solutions can increase the public’s trust in immunisation campaigns and therefore bolster vaccine acceptance. In 2019, the World Health Organization (WHO) released its list of the top 10 global health threats. Among the threats topping that list were another global influenza pandemic, Ebola and other high threat pathogens, and vaccine hesitancy. The WHO ...
Storing vaccines at the right temperatures has always been a challenge everywhere in the world, but especially so in remote areas. The new TCW80SDD Vaccine Refrigerator will allow to keep vital vaccines refrigerated in places without electrical infrastructure, making vaccinations more equitable around the world. Storing vaccines at the right temperatures has always been a challenge everywhere ...
The arrival of the COVID-19 pandemic has challenged the world’s health system and organization. To control and prevent this virus and others of its kind, vaccination has been adopted as an effective way to protect public health. Vaccines are chemical and biological products, which means they are sensitive to both cold and heat and must be kept in cold storage throughout the vast cold ...
All vaccines are temperature sensitive and require the proper environmental conditions to ensure vaccine stability. ...
For research facilities, hospitals, clinics, doctor offices and other medical establishments, vaccine storage is absolutely critical to ensuring the accuracy and efficiency of vaccines administered. Although regulatory organizations carry out standard audits to examine vaccine storage procedures, it is important ...
There are several potential COVID-19 vaccines that may soon be available for widespread distribution. In particular, the United Kingdom has recently approved Pfizer’s vaccine, and the U.S. Food and Drug Administration is considering extending Emergency Use Authorization to the Pfizer and Moderna vaccines. That is certainly promising news, but storage, transportation, and delivery of these ...
Unlike medicines, which are used to treat or cure diseases, vaccines are intended to prevent them. Handling and Storage of Vaccines Developing a vaccine can take years before it is deemed safe for human use and, thereafter manufactured and made available for widespread distribution and inoculation. ...
As Covid-19 counts continue to rise in North America, Europe and throughout the world, the potential for a vaccine comes at a critical time as scientists seek to prevent transmission of the virus by individuals already infected. On November 9, 2020, Pfizer and BioNTech announced that their trial of a Covid-19 vaccine showed a 90% efficacy rate and very promising results in participants who ...
Vaccine Storage and Handling Vaccines and other medical products are essential parts of a healthy society. ...
ByAKCP
All vaccines are sensitive to extreme heat and extreme cold and require both proper vaccine storage and management to avoid any loss of potency. Immunization is the most effective and economical public health measure against infectious diseases that cause morbidity or mortality. Immunization prevents two to three million deaths every year from diseases such as hepatitis, pertussis (whooping ...
ByAKCP
Medical Refrigerators are used to store temperature sensitive drugs and vaccines. Many vaccines require storage between 2°C and 8°C. Time spent outside of this temperature range are known as temperature excursions. Vaccines and drugs have something known as a drug stability budget, which is the total amount of time the substance can be kept outside of its recommended storage range. It ...
ByAKCP
For instance, the influenza vaccine may contain the genetic code of certain strains. The antigens from this vaccine are either weak or dead, so they do not cause diseases, unlike the virus. ...
ByAKCP