Broncus Medical, Inc. products
Navigation
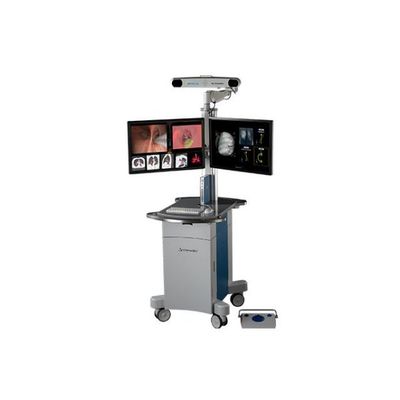
Archimedes - Virtual Bronchoscopic Navigation System
The Archimedes® Virtual Bronchoscopic Navigation System, also known as LungPro® in Asia Pacific regions, integrates CT, pattern recognition software, and fused fluoroscopy to provide three-dimensional, real-time Guided Transbronchial Needle Aspiration (TBNA) and proprietary parenchymal navigation, commonly referred to as Bronchoscopic Trans-Parenchymal Nodule Access (BTPNA). The planning software combines state-of-the-art nodule, vessel and airway mapping technology with virtual pathway guidance to ensure a safe and efficient Guided TBNA or BTPNA procedure. Archimedes is the only pulmonary navigation system that provides multiple, up to four (4), bronchoscopic techniques to access and diagnosis a nodule regardless of size, location or the presence of a bronchus sign, all common challenges associated with lower Diagnostic Yield (DY).
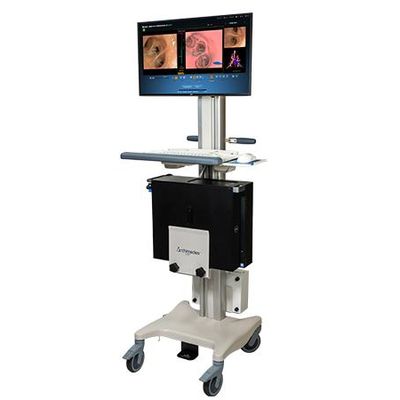
Archimedes - Virtual Bronchoscopic Navigation System
The Archimedes® Lite™ Virtual Bronchoscopic Navigation System, also known as LungPoint Plus® in Asia Pacific regions, is a simple, yet elegant virtual bronchoscopic navigation system that leverages the advanced navigation technology from Archimedes without fluoroscopy. Archimedes Lite is the only pulmonary navigation system that provides compatibility with any bronchoscope allowing for direct access to the lung periphery with NO dedicated procedure consumables, resulting in lower procedural costs. The clinical flexibility and low total cost of ownership make Archimedes Lite an ideal fit within any clinical practice.
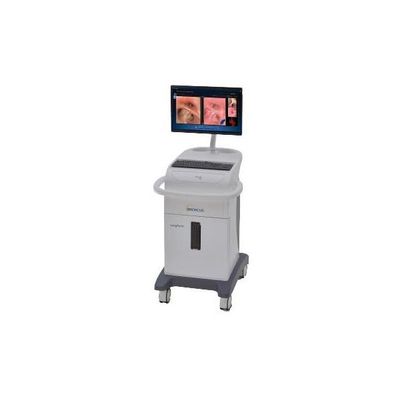
LungPoint - Virtual Bronchoscopic Navigation System
LungPoint, or LungPoint® Virtual Bronchoscopic Navigation, is a computer-assisted image-based navigation software system which, along with a set of biopsy tools, provides doctors with real-time path navigation within the airways and further localization guidance to a targeted area of interest in the lung for lung biopsy and other procedures. The system was designed to facilitate a higher biopsy diagnostic yield rate than that of any existing bronchoscopic biopsy modality by allowing for improved access to pulmonary peripheral lesions and nodules.
Diagnosis
BioStarNeedle - Single Use Ultrasound-Guided Aspiration Needle
BioStarNeedle is a single use ultrasound-guided aspiration needle for retrieval of tissue samples. Needle tip with nitinol materials provides excellent puncture with flexibility, while the special tip design offers precise retrieval. It obtained approval from Zhejiang MPA in June 2020, launch for sale in China.
FleXNeedle - Multipurpose 18g Biopsy Needle
Designed to enhance accessibility in hard-to-reach lesions in the peripheral segments of the lung: Shorter hypo tube length provides enhanced flexibility. Proprietary coring tip cuts and captures core specimens for histology and cytology. Needle internal lumen is 0.95mm enabling a large sample collection. Adjustable depth gauge provides penetration of 5mm to 20mm for precision and control. 1400mm catheter length is compatible with most extended working channels or guide sheath.
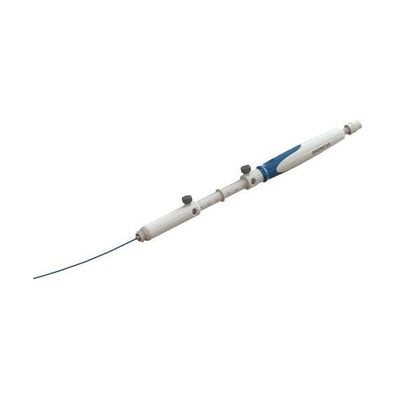
Archimedes - Sterile Single-Use Flexible Tube
The Archimedes® Sheath is a sterile single-use flexible tube that provides a working channel during a bronchoscopic procedure through which other devices may be introduced to the targeted area within the respiratory organs. The sheath is advanced through the working channel of a bronchoscope and allows for the repeated placement of endoscopic devices to a specified lesion(s) during a procedure. The Archimedes Sheath was cleared for marketing and commercial use in the U.S. by the FDA in 2013, the EU by the BSI in 2014, and the PRC by the NMPA in 2018.
Archimedes - Dilation Balloon for Sterile Single-Use Flexible Tube
The Archimedes® Balloon is a sterile single-use flexible tube with a balloon at or near the distal tip that is inserted through the bronchoscope or sheath and used to dilate the target lung tissue of the bronchial tree. The Archimedes Balloon was cleared for marketing and commercial use in the U.S. by the FDA in 2013, the EU by the BSI in 2014, and the PRC by the NMPA in 2018.
Broncus - Steerable Sheath Adjustment Handle
The steerable sheath consists of a steerable sheath adjustment handle and a working channel. The traction system of the adjustment handle can adjust the position and angle of the distal end of the sheath, so that the sheath can reach the lesion position accurately. The damping system of the adjustment handle can lock the angle, so that the sheath can stay at any position when the surgeon performs the bending operation. The working channel is connected with the adjustment handle through a connector.
Treatment
InterVapor - Minimally Invasive Bronchoscopic Treatment System
The InterVapor® System is a minimally invasive bronchoscopic treatment that is designed to deliver targeted Bronchoscopic Thermal Vapor Ablation (BTVA®) to ablate the most diseased hyper-inflated lung segments. The ablation results in a reduction in emphysematous tissue and volume in the targeted segment(s) allowing for healthy lung regions to expand and function more efficiently. BTVA is a non-surgical and non-implant therapy that has been proven effective in patients with collateral ventilation (CV+), can be performed under light sedation and typically takes less than 15 minutes. In patients with severe emphysema, vapor ablation of the most diseased areas of the lung may result in clinically meaningful improvement in breathing by increasing pulmonary function. In 2017, the InterVapor System received a CE Mark, allowing commercialization of the product in Europe.
Broncus - Radiofrequency Ablation System
RF-II is a radiofrequency ablation system used in conjunction with a disposable lung radiofrequency ablation catheter, which acts on lung tumors via a bronchoscope to perform ablation to the lung tumors. It is currently the only RFA system that specifically focuses on lung cancer treatment globally, according to Frost & Sullivan. According to Frost & Sullivan, radiofrequency ablation is the most widely used ablative technique globally for the treatment of lung malignancies.