Broncus Medical, Inc. products
Treatment
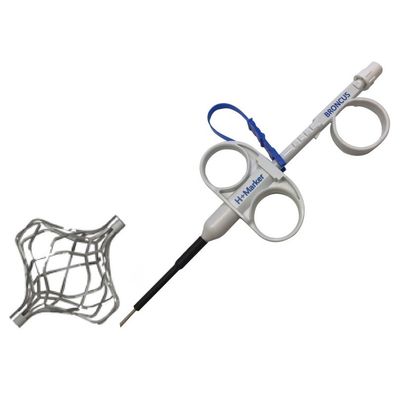
Broncus - Self-Developed Pulmonary Surgical Marker
Self-developed pulmonary surgical marker can be used to mark the location of the lung nodule to achieve precise positioning during surgical pneumonectomy. Compared with traditional methods, H-Marker can not only help surgeons to locate nodule on pleura but also to identify the depth of wedge resection. It obtained approval from Zhejiang MPA in June 2021, launch for sale in China.
InterVapor - Minimally Invasive Bronchoscopic Treatment System
The InterVapor® System is a minimally invasive bronchoscopic treatment that is designed to deliver targeted Bronchoscopic Thermal Vapor Ablation (BTVA®) to ablate the most diseased hyper-inflated lung segments. The ablation results in a reduction in emphysematous tissue and volume in the targeted segment(s) allowing for healthy lung regions to expand and function more efficiently. BTVA is a non-surgical and non-implant therapy that has been proven effective in patients with collateral ventilation (CV+), can be performed under light sedation and typically takes less than 15 minutes. In patients with severe emphysema, vapor ablation of the most diseased areas of the lung may result in clinically meaningful improvement in breathing by increasing pulmonary function. In 2017, the InterVapor System received a CE Mark, allowing commercialization of the product in Europe.
Broncus - Radiofrequency Ablation System
RF-II is a radiofrequency ablation system used in conjunction with a disposable lung radiofrequency ablation catheter, which acts on lung tumors via a bronchoscope to perform ablation to the lung tumors. It is currently the only RFA system that specifically focuses on lung cancer treatment globally, according to Frost & Sullivan. According to Frost & Sullivan, radiofrequency ablation is the most widely used ablative technique globally for the treatment of lung malignancies.