Refine by
Coronary Artery Bypass Graft Suppliers & Manufacturers
12 companies found

based inLivermore, CALIFORNIA (USA)
Advanced Bifurcation Systems has disruptive technology for left main heart disease, stenting coronary arteries at their branch points. This platform is a paradigm shift in interventional cardiology. The ABS platform is protected by more than 36 ...

based inCarlsbad, CALIFORNIA (USA)
CeloNova BioSciences, Inc. is developer and manufacturer of a portfolio of novel surfaced coated technologies, including the proprietary Polyzene-F nanocoating. This next-generation nanocoating is the result of years of rigorous scientific research ...
COBRA PzF NCS is the world’s first non-drug eluting, nanocoated stent that allows physicians to safely and effectively treat their patients who may benefit from a short, minimum 1-month DAPT ...

based inKiel, GERMANY
tiakis biotech AG (“tiakis”) – formerly Proteo Biotech AG - is the next generation preventive therapeutic technology company. We focus on our clinical-stage proprietary and pioneering tissue protective drug ...
Tiprelestat is a pioneering anti-inflammatory and tissue protective drug being developed for the treatment of pulmonary-arterial hypertension, the prevention of serious disease progression of COVID-19 and the prevention of postoperative inflammatory ...

based inConshohocken, PENNSYLVANIA (USA)
BTG Specialty Pharmaceuticals provides rescue medicines typically used in emergency rooms and intensive care units to treat patients for whom there are limited treatment options. We are dedicated to delivering quality medicines that make a real ...
We believe in the transformative power of medical innovation to improve human health. We set out to establish the safety and efficacy of our products, but also to improve standards of care. Ultimately, we want to help physicians better care for ...

based inLeeds, UNITED KINGDOM
Founded in 2009, Leeds-based Arterius is developing a next-generation bioresorbable cardiovascular scaffold (stent) with an emerging market-leading clinical profile. The founding directors have significant experience in the development of medical ...

based inStillwater, MINNESOTA (USA)
Vascudyne is developing, manufacturing and commercializing regenerative acellular in-vitro grown “allograft” tissue. We are visionaries and realists in the right proportions. We are after the holy grail of bringing tissue-engineered medical products ...
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic ...

based inWayne, PENNSYLVANIA (USA)
XyloCor Therapeutics is a biopharmaceutical company focused on the development of novel gene therapy for unmet needs in advanced coronary artery disease. In the United States, coronary artery disease is a leading cause of death and disability. Our ...
XC001 is an investigational gene therapy for the treatment of patients with refractory angina with no treatment options. Patients who have exhausted pharmacologic options and are not eligible for PCI or coronary ...

based inJena, GERMANY
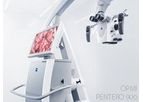
ZEISS is an internationally leading technology enterprise operating in the fields of optics and optoelectronics. In the previous fiscal year, the ZEISS Group generated annual revenue totaling 7.5 billion euros in its four segments Semiconductor ...
OPMI PENTERO 900 represents the next generation visualization system. Building on the innovations of OPMI Pentero introduced in 2004, OPMI PENTERO 900 combines award-winning design concepts and new functionalities in a proven, fully integrated ...

based inEindhoven, NETHERLANDS
Xeltis is a clinical-stage medical device company pioneering a restorative approach in cardiovascular therapy. RestoreX™, Xeltis technology platform, is the world’s first polymer-based technology designed to enable natural restoration of ...
The XABG device is a restorative, synthetic, electrospun blood vessels for CABG surgery, overtime turning into a living vessel made of patient’s own ...

based inMonmouth Junction, NEW JERSEY (USA)
MedChemExpress (MCE) offers a wide range of high-quality research chemicals and biochemicals (novel life-science reagents, reference compounds and natural compounds) for scientific use. We have professionally experienced and friendly staff to meet ...
Aprotinin is a bovine pancreatic trypsin inhibitor (BPTI) inhibitor which inhibits trypsin and chymotrypsin with Kis of 0.06 pM and 9 nM, ...

based inCarlsbad, CALIFORNIA (USA)
Palisade Bio is a late-stage biopharma company advancing oral therapies that help patients with acute and chronic gastrointestinal complications stemming from post-operative digestive enzyme damage. Our initial focus is guarding against the ...
Our lead asset LB1148 is a novel oral liquid formulation of the well-characterized digestive enzyme inhibitor, tranexamic acid (“TXA”), with potential to both reduce abdominal adhesions and help restore bowel function following surgery. ...

based inMason, OHIO (USA)
AtriCure is a medical device company that provides innovative solutions designed to decrease the global Afib epidemic. As a leading provider of innovative technologies for the treatment of Atrial Fibrillation (Afib) and related conditions, ...
The Isolator Synergy ablation clamp is the FIRST and ONLY surgical ablation device offered with FDA approval for the treatment of Atrial Fibrillation (Afib) and the only device either Catheter or Surgical to be approved for the treatment of ...