Show results for
Refine by
Locations
- USA
- Alabama
- Alaska
- Arizona
- Arkansas
- California
- Colorado
- Connecticut
- Delaware
- District Of Columbia
- Florida
- Georgia (Us)
- Hawaii
- Idaho
- Illinois
- Indiana
- Iowa
- Kansas
- Kentucky
- Louisiana
- Maine
- Maryland
- Massachusetts
- Michigan
- Minnesota
- Mississippi
- Missouri
- Montana
- Nebraska
- Nevada
- New Hampshire
- New Jersey
- New Mexico
- New York
- North Carolina
- North Dakota
- Ohio
- Oklahoma
- Oregon
- Pennsylvania
- Rhode Island
- South Carolina
- South Dakota
- Tennessee
- Texas
- Utah
- Vermont
- Virginia
- Washington
- West Virginia
- Wisconsin
- Wyoming
Covid 19 Surveillance Testing Suppliers Serving Usa
53 companies found

based inTaipei City, TAIWAN
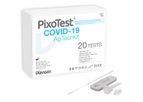
iXensor is the pioneer of mobile health. We turn ordinary smartphones into versatile lab-grade medical diagnostic devices for point-of-care testing and at-home self-testing solutions. PixoTest, our first product, is also the world’s first FDA ...
The PixoTest COVID-19 Ag Test is a rapid immunochromatographic assay for the qualitative detection of SARS-CoV-2 present in human nasopharynx. The PixoTest® COVID-19 Ag Test, together with PixoTest® POCT Analyzer (Model No. POC-X02), ...

based inRichmond, BRITISH COLUMBIA (CANADA)
Providing Timely PCR Covid Testing. Pacgen is a life sciences company focused on building a global commercial platform to market and distribute innovative consumer health products developed by small business enterprises in North America. We are ...
When it comes to COVID-19 testing, the choice of test is usually determined between accuracy and speed. An antigen test can provide results quickly, but a PCR test is considered the gold standard for COVID-19 testing. PCR testing can ...

based inShanghai, CHINA
Focusing on the core raw materials of the life science industry, Yeasen Biotechnology (Shanghai) Co., Ltd. is a biotechnology company engaged in the research and development, production and sales of three major categories of biological reagents: ...

based inEmeryville, CALIFORNIA (USA)
Lucira Health has developed an at home COVID-19 test kit that delivers PCR quality molecular accuracy in the palm of your hand in 30 minutes or less. Our technology is transforming how we reduce the spread of infectious diseases by bringing ...
The Lucira Check It is a molecular or nucleic acid amplification test (NAAT) that provides accurate, at-home results for the detection of the COVID-19 virus. Our tests have an analytical sensitivity (ability to detect the SARS-CoV-2 virus) ...

based inKowloon Bay, HONG KONG
Hybribio, a leader in molecular diagnostic products in China, is engaged in the nucleic acid diagnostic field. It provides a full operation chain from research and development to production and sales. The company's capabilities are enriched by an ...

based inMarlborough, MASSACHUSETTS (USA)
Hologic, Inc. is a medical technology company primarily focused on women’s health; it sells medical devices for diagnostics, surgery, and medical imaging. We’re an innovative medical technology company whose purpose is to enable healthier lives ...
The emergence of SARS-CoV-2 was unforeseen, and the worldwide outbreak of COVID-19 has already impacted millions. In response to the desperate public need for rapid high-volume testing we developed SARS-CoV-2 assays on both our Panther® and ...

based inSanta Clara, CALIFORNIA (USA)
LumiQuick Diagnostics Inc., located in the heart of Silicon Valley, California, USA, develops, manufactures and markets high quality point of care tests and other immunoassay kits for the world-wide In vitro diagnostic market. Through years of ...

based inAhrensburg, GERMANY
ulti med Products (Deutschland) GmbH specialises in the development, manufacture as well as worldwide export of high-quality In-vitro diagnostics and drug tests. The high quality standards applicable to our products and our quality management system ...
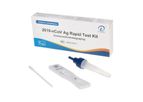
This kit is used for the in vitro qualitative detection of 2019-nCoV antigen. It is an immunochromatography sandwich assay, and intended to detect 2019-nCoV N-protein antigen in human nasal (NS) swab specimens. This kit can be used for individuals ...

based inZhengzhou, CHINA
Since 2014, DiaSino Laboratories specialized in design, development, manufacture and worldwide distribution of Immunodiagnostic reagents, including three platforms: Porrima fully automatic chemiluminescence immunoassay, Infinosis fluorescent ...
Nasal swab: Sensitivity: 95.38% Specificity: ...

based inWest Sacramento, CALIFORNIA (USA)
We founded Molecular Matrix in 2011. After seven years of rigorous research and development on our tissue regeneration technology, we received FDA clearance for our breakthrough bone regeneration medical device. We believe that medicine is a ...
Test for antibodies that neutralize SARS-CoV-2, the virus that causes COVID-19. This is the only FDA EUA approved test to determine whether you have functional neutralizing antibodies. Other IgG/IgM antibody tests do not provide results on the ...

based inDanyang City, CHINA
Jiangsu Konsung Bio-medical Science and Technology Co., Ltd established in 2005. Konsung has a production base in Danyang, Jiangsu, and R&D center in Shenzhen. The main products of Konsung includes the multi parameter monitor, mobile health monitor, ...

based inSouth San Francisco, CALIFORNIA (USA)
BioCheck, located in the San Francisco Bay Area, was founded in April 1997, to develop and manufacture high-quality enzyme immunoassay test kits for the worldwide biomedical, pharmaceutical and scientific research markets. The founders have brought ...

based inRedwood City, CALIFORNIA (USA)
Telomere Diagnostics, Inc. (TDx) is a privately held DNA testing company committed to helping people improve their health. TDx's lab in Silicon Valley (Redwood City, California) is CLIA-certified under the Clinical Laboratory Improvement Amendments ...
In addition to our current nasal testing offering, we are now offering saliva testing for COVID-19. Our saliva testing is a simple and pain-free process, and samples can be more easily provided as no medical personnel is required for collection. ...

based inTempe, ARIZONA (USA)
GenoSensor Corporation is a genomic technology company, aiming to improve human healthcare by developing and marketing products and services for genomic research, drug discovery, predisposition gene screening, therapeutic assessment and other ...

based inGlen Allen, VIRGINIA (USA)
GENETWORx is dedicated to improving the lives of all people, whether it’s through COVID-19 diagnostic or antibody testing, or pharmacogenomics. GENETWORx Laboratory is a health management service company that specializes in bringing the benefits of ...
COVID-19, influenza, and respiratory syncytial virus (RSV) are respiratory illnesses caused by different viruses. Because they have similar symptoms it can be difficult to tell them ...

based inGuro-gu, SOUTH KOREA
We provide diagnostic solutions throughout the cancer treatment cycle from early cancer diagnosis to prognostic and companion diagnostics as well as postoperative monitoring. Diagnose cancer as early as possible, when the disease is easiest to ...
What is this test? GenePro COVID-19 Detection Test is developed based on “WHO interim guidance for laboratory testing for 2019 novel coronavirus (SARS-CoV-2) in humans” by WHO (World Health Organization), and qualitatively detects RdRP ...

based inHouston, TEXAS (USA)
Elabscience specializes in immunodiagnostic technology for life science community. We have complete platform for R&D and manufacture. At the same time, we have in house QC for every product, endeavoring to keep your experiment results more ...
COVID-19 (Corona Virus Disease) is an infectious disease caused by the most recently discovered coronavirus. COVID-19 IgG/IgM Rapid Test ( Serum/Plasma/Whole Blood) is a rapid chromatographic immunoassay for the qualitative of IgG and IgM antibodies ...

based inHennigsdorf, GERMANY
Our company was founded by a group of scientists, who were confronted with the time-consuming process of human biospecimen procurement. Our experiences have motivated us to develop an online marketplace, that connects people in the life science ...
General Data: CBH Donor ID: CBHD131. CBH Master ID: CBHM131. CBH Sample ID: CBHS132. Matrix: serum. Storage Container: Cryotube (0.5 ml). Storage Temperature: < -18 degrees Celsius. Freeze Thaw Cycles: 0, Infectious Disease Test Result, ...

based inJinan, CHINA
Founded in 2003, Jinan Babio Biotechnology Co., Ltd. is a professional high-tech enterprise which concentrates on the R&D, manufacturing and selling of in vitro diagnostic reagent. The company was successfully listed on the NEEQ (National Equities ...

based inSan Diego, CALIFORNIA (USA)
Able Diagnostics, Inc. is a well established diagnostic and research company founded by a group of passionate and experienced diagnostic product professionals who want to make a difference. We focus on high quality and competitively priced Blood ...
The 1st Point-of-Care (POC) Antibody Test for COVID-19 with Fingerstick Whole Blood Authorized by FDA!. Click the link to review FDA NEWS RELEASE on Sept 23, 2020 in details: "FDA Authorizes First Point-of-Care Antibody Test for COVID-19" about ...