Refine by
Vascular Access Suppliers & Manufacturers
77 companies found

based inTraverse City, MICHIGAN (USA)
Thompson Surgical Instruments delivers Uncompromised Exposure to a variety of surgical specialties with the Thompson Retractor. In use for over fifty years, the Thompson Retractor is the only mechanical retractor to offer one frame for all exposure ...
The OneFrame Retractor Set by Thompson Surgical is a powerful tool designed for enhanced surgical access and retraction. It integrates seamlessly with the versatile OneFrame platform, delivering enhanced general and ...

based inBillerica, MASSACHUSETTS (USA)
Access Vascular is offering a suite of vascular access devices that use our novel, patented biomaterial featuring MIMIX™ technology which is designed to prevent the most common and costly complications in vascular access. Keep treatment moving ...
A peripherally inserted central venous catheter (PICC) is a central line used to gain vascular access with its tip extending to the superior vena ...

based inParis, FRANCE
emka TECHNOLOGIES, now joined with SCIREQ, has been providing integrated systems for preclinical physiology, pharmacology & toxicology research for over 25 years. Our hardware and software are used daily, validated by major pharma companies and CROs ...

based inLabège, FRANCE
Created in 1985 by Pierre Montoriol, Hemodia head office is located in Toulouse, in the south west of France. The company is known for its expertise in the design, production and marketing of single-use medical devices. Hemodia is a key player on ...
Care kits for: cannulation and line insertion, immediate or deferred infusion connection, medication reconstitution for drug delivery, filling of infusion devices, disconnection, needle withdrawing, maintaining the permeability of the ...

based inWorcester, UNITED KINGDOM
We are an innovative and trusted manufacturer and supplier of specialised procedure packs and vascular access devices to hospitals across the world. At Kimal, we are proud of our achievements but we never sit back on our laurels, as we strive to ...
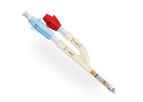
The Bioflo PICC with Endexo and PASV technologies provides safe and reliable mid to long-term intravenous access. ...

based inSeattle, WASHINGTON (USA)
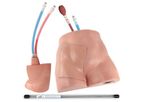
Since 1994, Simulab has been committed to providing medical simulators and task trainers to the medical education community. By collaborating with leading educators worldwide, leveraging its years of experience, and bringing a specialized knowledge ...
Simulab's FemoraLineMan Training Package is an ultrasound-compatible task trainer that offers an effective training solution for central venous or arterial access using the femoral site. This femoral line trainer uses the same patented ...

based inDoha, QATAR
QG Medical Devices is a leading manufacturer of Medical Devices in the Middle East. Headquartered in Doha, Qatar, QG Medical Devices boasts a state of the art production facility designed by reputable innovative technology providers from Germany, ...
Q Flow range of single use intravenous cannula is a holistic, durable and reliable medical device designed to accommodate all types of IV therapy. Our Q Flow products are designed with features and functionality built around the patients' and ...

based inPlano, TENNESSEE (USA)
Integer is a leader in advanced medical device outsourcing. Our story is one filled with 80 years of industry-changing innovations and exemplary manufacturing. It’s a legacy we are proud of as we continue to provide customers with unparalleled ...
With over 35 years experience and with product certifications in over 70 countries, Oscor is the industry leader in the design and development of minimally invasive and implantable medical ...

based inRedmond, WASHINGTON (USA)
The story of EchoNous (ek-koh-nohs) begins in Redmond, Washington with its founding in 2016 by point-of-care ultrasound pioneer Kevin Goodwin and Dr. Niko Pagoulatos, a recognized innovator in cutting-edge ultrasound and bioengineering. The name ...
A highly reliable, all-electronic, non-mechanical bladder tool designed to improve durability. EchoNous Bladder brings together data-rich fanning, a state-of-the-art AI algorithm using Convolutional Neural Networks (CNN) and a rugged probe design to ...

based inCarlsbad, CALIFORNIA (USA)
CeloNova BioSciences, Inc. is developer and manufacturer of a portfolio of novel surfaced coated technologies, including the proprietary Polyzene-F nanocoating. This next-generation nanocoating is the result of years of rigorous scientific research ...
COBRA PzF NCS is the world’s first non-drug eluting, nanocoated stent that allows physicians to safely and effectively treat their patients who may benefit from a short, minimum 1-month DAPT ...

based inLivani, LATVIA
Lightguide International is a dynamic and competitive European company that develops, manufactures and supplies optical fibers, fiber bundles, cables and laser delivery systems for cutting-edge, highly sophisticated scientific, industrial and ...
Lightguide’s Infinity Side Fiber is the next generation instrument for radial laser treatment. Recent innovations made by Lightguide result in our best laser fiber for soft tissue laser surgery. The broad cylindrical 360° laser light ...

based inWaldbuttelbrunn, GERMANY
In 2000, TauroPharm GmbH was founded as a German company specialising in medical devices with antimicrobial efficacy. We have since developed a variety of innovative products and built an international network. As TauroLock solutions have been ...
If you are looking for a catheter lock solution with both an antimicrobial and anticoagulant effect, we recommend TauroLock™. On this site, you can find detailed information about the product, its active ingredients, and ...

based inLatham, NEW YORK (USA)
AngioDynamics, with its disruptive and innovative medical devices, addresses unmet patient needs by elevating the standard of care for chronic and acute disease states in vascular, peripheral vascular, and oncology medicine. These devices provide ...
The “next generation” DuraFlow 2 chronic hemodialysis catheter from AngioDynamics continues to deliver the performance-tough features you demand and the care your patient deserves. Building on the standards for high performance, strength ...

based inStoughton, MASSACHUSETTS (USA)
SyncMedical LLC develops, produces and markets medical devices exclusively for regions outside of the United States by leveraging its strategic partnerships with industry leaders and medical professionals. Our focus is on developing access to the ...
The Primo Port is a fully implantable vascular access device that meets the requirements of clinicians and patients by providing long-term repeated access to the vascular system. ...

based inBirmingham, ALABAMA (USA)
With Rampart, interventionalists across the nation and around the globe can finally experience comprehensive, apron-free radiation protection—all so they can do what they are passionate about and what they do best - in a safe and team oriented ...

based inSan Francisco, CALIFORNIA (USA)
Velano Vascular is transforming the inpatient experience with PIVO a breakthrough needle-free blood collection device. PIVO addresses the fear, pain, and anxiety associated with traditional venipuncture blood draws while improving practitioner ...

based inChambly, FRANCE
ISOMed is specialized in disposable medical devices for vascular access. Since 1990, we propose therapeutic solutions in vascular surgery, oncology and digestive surgery. Our products meet the technical requirements practitioners demand everywhere ...
ISO Med offers, under the FB Medical brand, a complete range of Huber Needles, straight or 90° curved. They are designed for single use and easy insertion into vascular access device, and may be used for ...

based inAlpharetta, GEORGIA (US) (USA)
Founded by Fred L. Aycock in 1992, Remington Medical Inc. specializes in medical devices. The maintenance, time, and money put into cleaning and sterilizing medical devices led us to develop some of the first disposable cables, some still in use ...

based inMinneapolis, MINNESOTA (USA)
At Medtronic, we believe in the power of medical technology to improve lives. Seven decades ago, our co-founder invented the battery-powered pacemaker. Today, we are among the largest medical device companies in the world. With operations in 150 ...
The Crescent™* catheter is the first FDA-cleared, jugular dual lumen long-term ECMO catheter. It allows for more accurate placement with just one cannulation site, delivers enhanced flow dynamics, and helps maintain optimal flow† once ...

based inCantù (CO), ITALY
Bioengineering Laboratories (BEL) has been conceived as a young, streamlined and multi-faceted organization able to provide technical knowledge and a twenty year experience in designing, testing and producing innovative medical devices in the ...