- Home
- Companies
- asia middle east
- clinical trials
Show results for
Refine by
Locations
- Asia & Middle East
- Afghanistan
- Armenia
- Azerbaijan
- Bahrain
- Bangladesh
- Bhutan
- Brunei Darussalam
- Cambodia
- China
- East Timor
- Georgia
- Guam
- India
- Indonesia
- Iran
- Iraq
- Israel
- Japan
- Jordan
- Kazakhstan
- Kuwait
- Kyrgyzstan
- Laos
- Lebanon
- Malaysia
- Maldives
- Mongolia
- Myanmar
- Nepal
- North Korea
- Oman
- Pakistan
- Palestinian Territories
- Philippines
- Qatar
- Russia
- Saudi Arabia
- Singapore
- South Korea
- Sri Lanka
- Syria
- Taiwan
- Tajikistan
- Thailand
- Turkmenistan
- United Arab Emirates
- Uzbekistan
- Vietnam
- Yemen
Clinical Trials Suppliers In Asia Middle East
3 companies found

based inAhmedabad, INDIA
Founded in the year 1983. Troikaa Pharmaceuticals started its first commercial activities in 1984 by transforming a cotton ginning mill into a pharmaceutical unit in Thol village with just 15 people. Our beginnings were humble, but we dreamed to ...
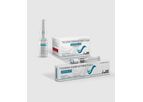
Dynapar AQ is a novel Diclofenac injection manufactured by the Aquatech Process, which provides the full dose of 75 mg Diclofenac in just 1 ml. The Aquatech process provides the optimal concentration of Diclofenac in an aqueous solvent system, ...

based inWaltham, MASSACHUSETTS (USA)
Thermo Fisher Scientific Inc. (NYSE: TMO) is the world leader in serving science, with annual revenue exceeding $25 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are ...
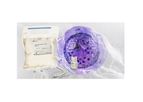
Natural killer (NK) cells are innate lymphocytes that kill virally infected or malignant cells. Unlike T cells, NK cells function in an antigen independent manner, responding to anything they perceive as "non-self", including malignant cells. NK ...

based inKraainem, BELGIUM
With over 17,000 staff in around 200 laboratories across 36 countries, Eurofins Scientific is the world leader in food and biopharma product testing. It is also number one in the world in the field of environmental laboratory services and one of the ...
Complete preclinical service: Early and full drug development is regulatory-driven and complex. The priority in this selection process is to achieve the safety and efficacy of a new molecular entity. The preclinical activity includes mainly safety ...